

A chelate, or more properly, a chelating agent, is a compound like EDTA, chlorophyll or hemoglobin that has niches in its ring structure for heavy metals like iron and copper. It attracts and holds the metal ions so tightly that they lose their normal characteristics, such as the ability to catalyze oxidation or form a precipitate. The process of attracting and holding is sometimes called "sequestration."
Anything that can prevent oxidation is sure to be used as a preservative, and such is indeed the case here. In the food industry sequestering agents aid in stabilizing color, flavor and texture.
DTPA, a chelating agent useful for the manufacture and preservation of paper, has been the subject of investigation in two disparate settings recently: a conservation research laboratory in Russia, and the lab of a commercial supplier of chelates in America.
The first research was reported in the Preprints of last September's ICOM meeting in Copenhagen.1 Newsprint, offset paper and 100% rag paper were both made and treated in different ways, then heat aged in closed glass tubes to show the effects of deacidification, tap vs. Distilled water, and DTPA alone and in combination with other conditions. Strength (folding endurance), color (brightness) and pH were all measured at 3-day intervals. DTPA, even when used alone, had a startlingly beneficial effect on all three kinds of paper. One would of course expect DTPA to protect newsprint, which deteriorates mainly by oxidation, but look at the results for 100% cellulose (sometimes called "rag") paper in Graph 0:
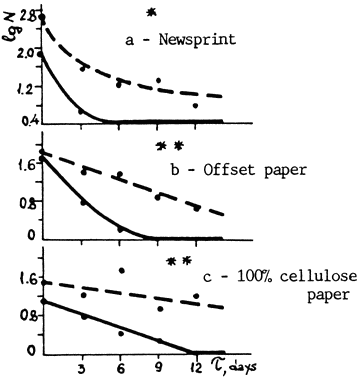
Fig. 1. Logarithm of the number of double folds (1g N) vs. time of accelerated aging (T).
| ___ | Untreated |
| ---- | Treated with DTPA solution |
| * | Measurement made under load 3.9 N |
| ** | Measurement made under load 6.9 N |
When used together with calcium and magnesium bicarbonate in solution, it made the paper four times as strong after 12 days in the oven as it would have been after deacidifying alone. It also kept the pH 1½ points higher than the pH for paper that was only deacidified. Here is the graph showing the effect of the various treatments on strength and pH:
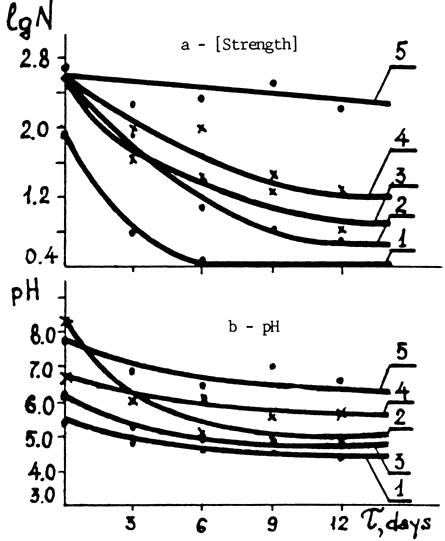
Fig. 4. Changes in the properties of newsprint subjected to various types of stabilization processes vs. time of accelerated aging (T).
a - Logarithm of the number of double folds (1g N)
b - Water extract pH
1. Untreated paper.
2. Paper treated with Ca and Mg bicarbonates.
3. DTPA solution treatment.
4. Ca - DTPA treatment.
5. DTPA + Ca and Mg bicarbonate treatment.
The second report, which appeared in the June Tappi Journal,2 describes a problem in the bleaching of pulp: heavy metals from the wood itself, especially iron, copper and manganese, rapidly break up the hydrogen peroxide bleach into oxygen and water before it has a chance to do its job properly, which means that larger quantities have to be used. This effect can be brought under control by using a sodium salt of DTPA in combination with sodium silicate and magnesium sulphate to precipitate the metal compounds formed. (This is not news actually. The purpose of the article was to give two case studies and some cost savings figures.)
DTPA stands for diethylenetriamine pentaascetic acid.
1. H.G. Blank and S. A. Dobrusina, "Raising of the Book-Paper Longevity by Means of Chelates and Ca-Chelate Compound." 84.14.13-84.14.15. The authors are at the M. E. Saltykov-Shchedrin State Public Library, 18 Sadovaja Street, 191069 Leningrad USSR.
2. D.R. Bambrick, "The Effect of DTPA on Reducing Peroxide Decomposition." Tappi Journal 68 (6): 96-100.