THE INFLUENCE OF DEACIDIFICATION ON THE DETERIORATION OF PAPERJ.S. Arney, A.J. Jacobs, & R. Newman
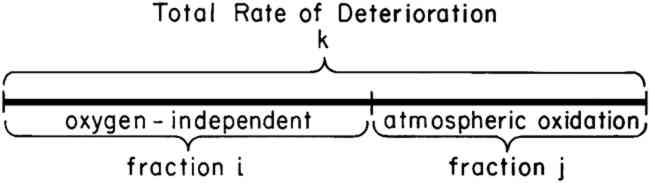
ABSTRACT—The influence of calcium carbonate deacidification on the rates of deterioration of commercial rag and newsprint papers at 90�C and 100% R.H. has been examined. Evidence is presented to show that the decreased rate of deterioration resulting from deacidification cannot be accounted for in terms of a decrease in the acid-catalyzed hydrolysis process alone. Atmospheric oxidation, shown to play an important role in the deterioration of the paper samples, is also retarded by deacidification. 1 INTRODUCTIONACIDITY HAS LONG BEEN RECOGNIZED as a major factor contributing to the deterioration of cellulose containing materials. In an effort to combat the harmful influence of acidity, investigators have developed a variety of deacidification techniques capable of decreasing the acid content of most works on paper that are to be found in museums and libraries.1 These techniques have been widely applied by conservators in the care of books and works of art on paper. However, deacidification is not advisable for all papers, and conservators are required to make judgments about whether to deacidify and in what way to deacidify each object entrusted to their care. In making these judgments, a myriad of factors must be considered, and in some cases insufficient information is available about the influence of deacidification to allow an informed judgment to be made. The research conducted in our laboratory has been undertaken in an effort to add to our understanding of the influence of deacidification on paper-containing objects. We have not, of course, been able to address all of the questions that have been raised by the conservation community about deacidification. We have, however, begun to explore the fundamental reasons why deacidification decreases the rate of paper degradation. The fundamental question asked in our research is: “Why is deacidification beneficial to paper?” The simplest answer is that deacidification neutralizes acids in paper, and acids have been known for nearly a century to be harmful to cellulose-containing materials.2 However, knowing that acids are harmful is not the same as understanding why acids accelerate aging or why deacidification increases paper stability. On the basis of the known chemical reactions of cellulose, it has been suggested that the aging of paper is a result of hydrolysis reactions and that acids catalyze the hydrolysis.3,4 However, recent publications have shown that an entirely different process, atmospheric oxidation, may also play a major role in the aging of some papers.5,6,7 The possible occurrence of both oxidative and hydrolytic processes in the aging of paper makes it difficult to predict the influence of deacidification. Although a decrease in acidity will decrease the rate of cellulose hydrolysis, the influence of pH on the oxidation of cellulose is poorly understood and differs for different oxidizing agents. For example, it is known that the oxidation of cellulose with hypochlorite is inhibited both by alkali and by acids.8 The influence of acidity on the oxidation processes that occur during the aging of paper has not been explored. The objective of the research described below was to determine this influence. 2 PRESENTATION OF RESULTSIN A PREVIOUS PUBLICATION, we have described a series of accelerated aging experiments that allow one to determine the contribution of atmospheric oxidation to the total rate of paper deterioration.6,7 The results of these experiments may conveniently be represented as shown in Figure 1 in which the total rate of degradation is shown as the length of the line labeled k. The percentage of this total rate that results from attack by oxygen is shown as fraction j. The remaining segment of line k has been labeled as oxygen-independent degradation, fraction i, and represents those processes, such as hydrolysis, that do not involve atmospheric oxygen. The reader is referred to previous publications6,7 for descriptions of the experimental procedures used to determine the values of i and j.
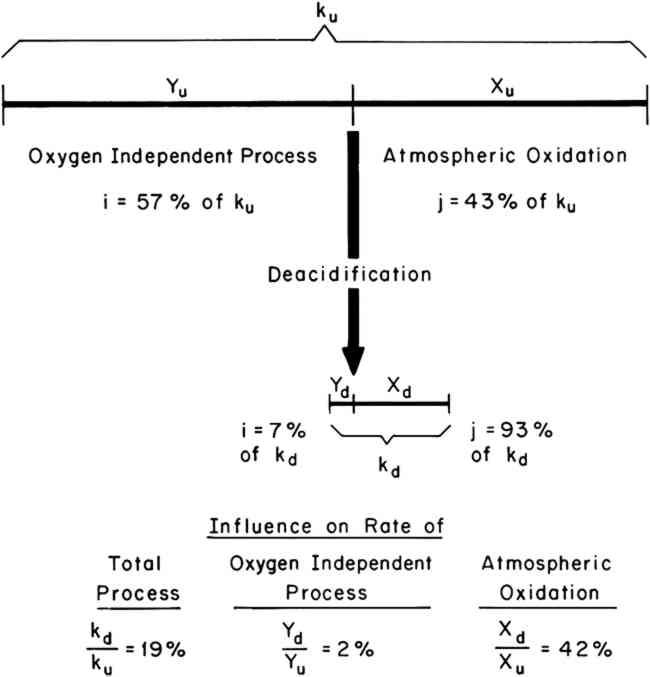
Values of the fractions i and j, expressed as percentages of the overall rate, k, have been determined for both a 100% cotton rag paper and a newsprint subjected to accelerated conditions of aging at 90�C and 100% R.H. From previously reported experiments,6,7 it was found that the 100% R.H. condition resulted in approximately equal contributions from the atmospheric oxidation and oxygen-independent processes. Therefore, in order to examine the influence of deacidification on both processes, the 100% R.H. condition was chosen for the accelerated aging experiments. Values of i and j for both types of paper were determined both with respect to the rate of loss of tensile strength and the rate of change in reflectance at 500 nm. Experiments were performed both on untreated papers and on deacidified papers in order to determine the influence of deacidification on the fractions i and j. The results are summarized in Table I. Table I Relative Contribution of Oxygen-Independent (i) and Atmospheric Oxidation (j) Processes to the Total Rate of Degradation at 90�C and 100% R.H. Before and After Deacidification As an example, consider the rate of tensile strength loss in the rag paper. Prior to deacidification it was determined that 57% of the rate of tensile strength loss was caused by oxygen-independent processes (iu = 57%), and 43% was caused by atmospheric oxidation (ju = 43%). After deacidification, values of id = 7% and jd = 93% were obtained for the rag paper. Thus, the effect of deacidification on the rate of tensile strength loss was to increase the relative importance of atmospheric oxidation and decrease the relative importance of the oxygen-independent process. One must exercise caution in the interpretation of these results and recall that the values of i and j for any single paper must always add up to 100%. Referring to Figure 1, an increase in j and a decrease in i simply means that the dividing point between i and j was shifted to the left. Whether the rate of atmospheric oxidation was increased or not cannot be determined from the data in Table I alone. One must also determine the influence of deacidification on the total rate of degradation; the length of line k. Experimentally, the length of kd (total rate of degradation of the deacidified paper) divided by the length of ku (total rate of degradation of the untreated paper) can be determined by dividing the time required for the untreated paper to reach a given level of degradation by the time required for the deacidified paper to reach the same level of degradation.9,10 Thus, values of kd/ku were determined for both the yellowing and the loss of strength of both the rag and the newsprint papers. The results are summarized in Table II. For example, the value of kd/ku is 19% for the loss of tensile strength in the rag paper. This means that after deacidification the rag paper lost strength 19% as fast as before deacidification. Thus the length of the line marked k in Figure 1 decreased significantly. Table II Experimental Values of the Rate (kd) of Degradation of Deacidified Paper Divided by the Rate (ku) of the Untreated Paper The data in Tables I and II may be understood by referring to the illustration in Figure 2. The length of the two horizontal lines represent the total observed rates of embrittlement, ku and kd, of the untreated and deacidified rag papers. The two lines are divided, as discussed above, into the two fractions of the deterioration process, i and j, of lengths X and Y. From this schematic illustration it can be seen that the rate of the oxygen-independent process (the length of line segment Y) is markedly decreased as a result of deacidification. In addition, it can be seen that the rate of the atmospheric oxidation process (the length of line segment X) is also decreased. Furthermore, the length of each line segment (Xu, Yu, Xd, and Yd) can be
A similar graphic treatment of the remaining data in Tables I and II resulted in the values of Xd/Xu and Yd/Yu reported in Table III. Table III Calculated Influence of Deacidification on the Rates of the Atmospheric Oxidation and Oxygen-Independent Processes in Rag and Newsprint Papers at 90�C, 100% R.H. 3 DISCUSSION AND CONCLUSIONSAS EXPECTED, deacidification of the rag and newsprint papers resulted in a significant decrease in their rates of yellowing and embrittlement during accelerated aging, as shown by the data in Table II. Results of this kind have often been reported in the technical literature, and the beneficial effect of reducing the acidity of paper is generally accepted. However, the assumption that the beneficial effect of deacidification is simply a result of a decrease in the rate of acid-catalyzed hydrolysis appears to be an oversimplification. Atmospheric oxidation, as well as the oxygen-independent component of deterioration, was retarded by deacidification, as shown by the data in Table III. The division of the deterioration process into an atmospheric-oxidation fraction and an oxygen-independent fraction is experimentally convenient but somewhat arbitrary. It is possible, and perhaps probable, that each of these fractions of the deterioration process is a result of more than one chemical sub-process. Although acid-catalyzed hydrolysis may be a sub-process that makes up part of the oxygen-independent fraction of deterioration, it would seem likely that other sub-processes are also involved. The two different values of Yd/Yu obtained for the rag paper by following the change in reflectance and the change in tensile strength, for example, suggest that the oxygen-independent processes leading to yellowing and embrittlement are somewhat different. In conclusion, the influence of acidity on the thermally induced deterioration of paper appears to be more complex than has previously been assumed, and the way in which deacidification treatments may alter the various deterioration processes is, as yet, not thoroughly understood. Many questions remain to be answered before the maximum benefit of deacidification is likely to be realized. What are the optimum pH values for regarding the various processes that contribute to deterioration? How does acidity influence the light-induced deterioration of paper? What are the possible roles of the common metal ions associated with the various deacidification techniques? Answers to questions such as these must be sought if optimum conditions for deacidification of the many types of paper found in museums and libraries are to be developed. 4 EXPERIMENTAL
APPENDIX1 APPENDIX1.1 Precision of Determination of Xd/Xu and Yd/YuALTHOUGH THE GRAPHIC METHOD of determining Xd/Xu and Yd/Yu is theoretically sound and intuitively easy to understand, it does not allow a rigorous analysis of the precision of the determinations. A more suitable analysis of the data involves equations (1) and (2). These equations may be derived from the geometry of Figure 2 or from the kinetic model suggested in our previous publication.7 From these equations, the data in Table III may be calculated directly. In addition, an analysis of variance of the data in Tables I and II (not shown) allows an error estimate of the data in Table III. The error limits shown in Table III are the 95% confidence intervals assuming a gaussian error distribution of Xd/Xu and Yd/Yu.
1.2 Speculation on Mechanisms of DegradationTHE TERMS “oxygen-independent” and “atmospheric oxidation” describe experimental manifestations only. They do not define uniquely the mechanism of degradation. In a kinetic study of this kind, one measures only the rate controlling process. Thus, the “atmospheric oxidation” process may involve any chemical mechanism whose overall rate is determined by reactions with oxygen. For example, an oxidative tendering of cellulose, catalyzed perhaps by transition metal impurities,5 might be followed by a fast hydrolysis. Such a mechanism would manifest itself as “atmospheric oxidation” if the oxidative tendering were, as one might suspect, rate controlling. Nevertheless, the actual loss of strength might be a direct result of the follow-up hydrolysis reaction. Numerous mechanistic scenarios of this kind might be envisioned that would be consistent with the kinetic data. Thus, one should not consider the determination of i and j as mechanistically conclusive. ACKNOWLEDGEMENTSTHIS INVESTIGATION was made possible by grants from the Andrew W. Mellon Foundation and the National Endowment for the Arts. The authors also express their thanks to Dr. R. L. Feller, Director of the Center on the Materials of the Artist and Conservator, for invaluable consultation and discussion. REFERENCESRecent discussions and bibliographies on deacidification techniques are to be found in: (a) “Preservation of Paper and Textiles of Historic and Artistic Value,” Adv. in Chem. Series No. 164, edited by J. C.Williams, ACS, 1977, and (b) Anne F.Clapp, “Curatorial Care of Works of Art on Paper,” 3rd edition, Intermuseum Conservation Association, Oberlin, Ohio, 1978. A.Girard, Ann. Chem., Phys., 24, 337, 382 (1881). R. D.Smith, The Library Quarterly, 39, 153 (1969). R. D.Smith, Adv. in Chem., Series No. 164, 149 (1977). J. C.Williams, C. S.Fowler, M. S.Lyon, and T. L.Merrill, Adv. in Chem., Series No. 164, ACS, 1977, p. 37. J. S.Arney and A. J.Jacobs, Preprints, p. 3, AIC Annual Meeting, Fort Worth, Texas, June, 1978. J. S.Arney and A. J., Jacobs, Tappi, 62(7), 89 (1979). L. F.McBurney, “Degradation of Cellulose,” High Polymers, Volume V, Part I, p. 142, Interscience Publishers, Inc., NY, 1954. A.Frank, Chemiker Ztg. Chem. Apparatur, 86, 174 (1962). R.Sizmann and A.Frank, Chemiker Ztg. Chem. Apparatur, 87, 347 (1963).
 Section Index Section Index |