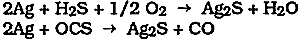A COMPARATIVE STUDY OF SILVER CLEANING ABRASIVESGLENN WHARTON, SUSAN LANSING MAISH, & WILLIAM S. GINELL
2 POLISHING CONSIDERATIONSPURE, CLEAN silver and many silver alloys are readily tarnished on exposure to the environment. Tarnishing is due to the reaction between silver and hydrogen sulfide (H2S), carbonyl sulfide (OCS), or various other sulfur-containing organic compounds in the atmosphere to form silver sulfide according to the following simplified overall reactions:
The rates of these reactions are strongly dependent on temperature, concentration, and relative humidity (Backlund et al. 1966; Bennett et al. 1969; Drott 1959; Franey 1983; Graedel et al. 1981; Graedel et al. 1985; Pope, Gibbens and Moss 1968). In the case of sterling silver, the tarnish layer contains cuprous sulfide (Cu2S), which may be distributed inhomogeneously depending upon the composition and distribution of the silver-copper phases in the alloy. A polished, mirrorlike surface is usually defined as one that reflects light specularly. For such an ideal surface, no light scattering occurs and all light incident on the surface is reflected at the incident angle. Scratches on a polished surface can be seen because they scatter the incident light. They will not be visible if the scratches are shallow, less than half the visible light wavelength (∼0.2 micrometers), or if the scratches are closer together than the light wavelength (∼0.4 micrometers). The objective of polishing, then, is to produce a surface whose irregularities are small with respect to the wavelength of visible light and not necessarily one that is featureless on an atomic level. Removal of the tarnish layer and polishing of the silver surface can be considered to be independent processes. An ideal cleaning system for silver is one that will remove the tarnish layer yet modify the underlying silver surface as little as possible. Such a system minimizes both the removal of silver and the alteration of the existing scratch pattern. In order to understand the mechanisms of polishing, the terms “abrasive polishing,” “soft material polishing,” and “burnishing” require explanation. Abrasive polishing involves the removal of surface material by the sharp cutting edges of abrasive particles; the topography of the resulting surface consists of a distribution of V-shaped grooves (Samuels 1982). The rate of material removal is determined by the number of abrasive particles in contact with the surface, the applied load, and the shape and rake angle of the particles. In some cases, depending on the rake angle, particles will plow a groove such that displaced metal forms a ridge on each side of the groove, but a significant amount of metal is not removed. The width and depth of the grooves are determined by both the particle size and applied load. For particle sizes less than a few tenths of a micrometer and with small loads, abrasives can produce a mirrorlike, polished surface. Phase contrast microscopy of such surfaces reveals a network of fine scratches (grooves) that are not visible at the same magnification by microscopy using ordinary illumination (Samuels 1978). Polishing by soft materials that are not ordinarily considered to be abrasive may occur by a different mechanism. If a scratched surface, consisting of hills and valleys, is rubbed by such materials, profilometer measurements show that the tops of the hills are gradually worn away until only the valley floors remain (Rabinowicz 1968). (A profilometer is an instrument whose active element is a fine-tipped stylus that is drawn across a surface and produces a record of the surface contour). This process produces an essentially featureless surface that appears polished. Although this process is also abrasion, it proceeds as an atom-by-atom material removal process rather than by mass removal by a cutting action. Metals that can be polished by this method seem to have low heats of vaporization, Hv. The rationale for correlation of this form of polishing with the Hv of a metal is that both processes involve removal of atoms from a surface. Because Hv for silver is moderately low, it is suspected that this is the primary polishing mechanism for soft abrasives such as calcium carbonate. Burnishing, another process that can result in a mirrorlike finish, can occur when a firm, usually hard, material is rubbed across a metallic surface. Inelastic metallic flow occurs, which results in metal transfer from high to low areas on the surface. On a microscale, the flow of metal modifies the original surface characteristics. Therefore polishing techniques that can produce burnishing should be used with caution on museum silver. The carrier fluid used with abrasives can act as a lubricant, a coolant, a vehicle for particle suspension, and a medium to flush debris away from the polishing site. Under conditions of hand polishing, silver will not reach a high temperature and hence the fluid is not required to perform a cooling or lubrication function. For silver polishing, the fluid alters the manner in which abrasive particles are dispersed among the yarns of the abrasive support cloth and assists in flushing away abrasive, tarnish, and silver particles. It has been found, in general, that polishing rates change significantly with different fluids (Samuels 1978). Either too much or too little fluid will reduce polishing efficiency, and therefore fluid evaporation during polishing may be a significant factor. Both the hardness of the abrasive support cloth and structure of the yarns play roles in determining polishing efficiency. Soft cloths provide greater contact area for the abrasive particles than hard cloths owing to the structure and weave of the cloth. Over time, abrasive particles become embedded in the cloth immediately in contact with the silver surface. These particles may or may not enhance polishing action. An agglomeration of metallic and tarnish particles may clog and cover the real areas of contact of the cloth, rendering the cloth less effective. If excessive amounts of debris accumulate, the cloth surface may take on the properties of a burnisher and smearing, rather than abrasion, may occur. The load applied during polishing also has an influence on the ultimate surface characteristics of the silver. Because the load applied to an abrasive particle embedded in the yarn of a soft cloth is light, only a small fraction of the particle thickness will penetrate the silver. This penetration will produce a narrow, shallow groove. The surface will appear polished, as the fine scratch pattern is below the level of visual detection. |