FUNGICIDAL EFFICACY OF SELECTED CHEMICALS IN THYMOL CABINETSRALPH A. GUSTAFSON, INGRID R. MODARESI, GEORGIA V. HAMPTON, RONALD J. CHEPESIUK, & GLORIA A. KELLEY
2 MATERIALS AND METHODS2.1 CABINET CONSTRUCTIONTWO IDENTICAL thymol cabinets (fig. 1) were constructed with �-in exterior-grade plywood following published specifications (Byers 1983; Nagan and McCann 1983). All joints were sealed with silicon caulking, and the interiors were painted with two coats of epoxy paint (Byers 1983). All doors and venting apertures were sealed with rubber gasket material. Two removable slat shelves were fitted above a solid �-in hardboard divider separating the fumigation chamber from the heat source. Three 10-cm diameter holes were cut into this divider, one above each light bulb, to accommodate 15-cm diameter watch glasses. The heat sources in each cabinet were three 25-W or 40-W Philips long-life light bulbs. One cabinet was vented to the outside through a building exhaust
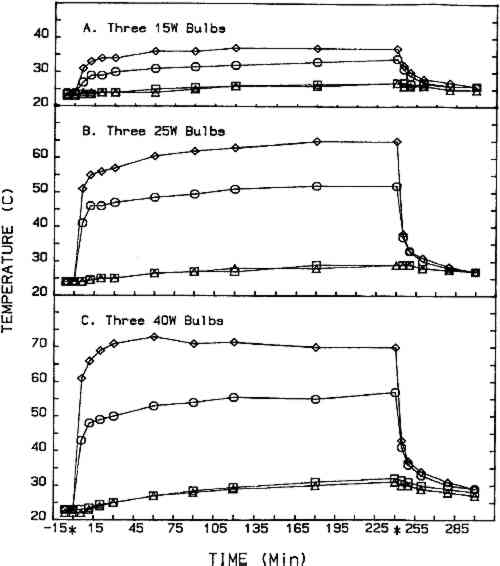
2.2 CHEMICALSThe eight chemicals evaluated in this study are described in table 1. Five grams of the chemical to be evaluated were placed in each watch glass (15 g total) for each fumigation cycle. Two chemicals, the parabens, are cosmetic industry preservatives, and their inclusion in this study was suggested by a cosmetic chemist. The remaining six chemicals are considered to have fungicidal activity (Windholz 1983). TABLE 1 Chemicals Evaluated 2.3 TEST ORGANISMSFour molds isolated from books in the Archives of Dacus Library were used throughout this study as the test organisms. They were Aspergillus niger, Penicillium sp. Cladosporium sp., and Emericella nidulans. The first three are Deuteromycetes and produce only asexual spores (conidia), and the last is an Ascomycete that produces both conidia and more resistant ascospores (sexual reproduction). 2.4 TREATMENT CYCLEEach fumigation cycle lasted 96 hours, with the timer-operated heat source (three light bulbs) on for the first 4 hours of each 24-hour segment. At the end of the 96-hour cycle, the treatment cabinet was exhausted to the atmosphere for 30 minutes prior to opening the doors. Treatment cycles for each chemical, except salicylic acid, were repeated at least once using 25-W bulbs, and one trial was completed for four chemicals using 40-W bulbs. Fresh cultures and new books were prepared for each treatment cycle. The control cabinet was fitted with four temperature probes: one directly beneath the center watch glass, one in the center watch glass, one in the center of the cabinet, and one at the top center of the cabinet. Cabinet temperatures during the 4-hour heating period for l5-W, 25-W, and 40-W bulbs are shown in figure 2. The control incubator was maintained at 25�C.
2.5 EFFICACY TESTINGThree procedures were designed to measure the fungistatic-fungicidal activity of each chemical. The first, colonial growth rate, measured the ability of each chemical to inhibit growth (fungistatic) or kill the mold (fungicidal); the second, spore germination inhibition, measured the chemical's ability to prevent germination of mold propagules; and the third evaluated the chemical's ability to kill indigenous molds on mildewed books. 2.5.1 Colonial Growth ExperimentsCultures of each mold for these experiments were spot-inoculated on Sabouraud dextrose agar plates 72 hours prior to the start of each treatment cycle. The inoculum 2.5.2 Spore Germination ExperimentsImmediately prior to the start of a treatment cycle, O.1 ml of a sterile phosphate buffer spore suspension of each organism was spread-plated on Sabouraud dextrose agar plates, and the lids on the treatment cabinet and control cabinet plates were replaced with sterile drilled lids. Five replicate plates of each organism were placed, inverted, in the treatment cabinet and control cabinet. Three replicate plates of each organism were placed in the control incubator. At the end of the 96-hour treatment cycle, the drilled lids were replaced with sterile intact lids, the plates were evaluated for growth or no growth, and all plates were then placed in the control incubator for 96 hours of post-treatment incubation. Following post-treatment incubation the plates were again evaluated for growth or no growth, and all control incubator, treatment cabinet, and control cabinet spore germination plates were subcultured onto fresh Sabouraud dextrose agar plates for viability determinations. Viability plates were evaluated for growth after 72 hours of incubation in the control incubator. 2.5.3 Book Sampling ExperimentsThese experiments involved two sampling procedures—agar overlays and swabbing. The Sabouraud dextrose agar overlay procedure was started 72 hours prior to the start of a treatment cycle. A sterile Petri dish bottom with an 8-mm diameter hole drilled in the center was inverted and taped to the top half of the front cover of each book. Fifteen ml of molten, tempered (46�C) Sabouraud dextrose sugar were pipetted onto the book cover within the dish. The hole was taped closed, and, after the agar solidified, the books were incubated in the control incubator at 25�C for 72 hours. Prior to starting the treatment cycle, the overlays were assessed for growth or no growth and the agar overlays removed from the books. The swab procedure was conducted immediately prior to starting the treatment cycle. Each book was sampled by swabbing a sterile, phosphate buffer moistened cotton-tip swab over a 9 cm2 area of the front, top left-hand corner of the book adjacent to the spine. After swabbing, the swab was used to inoculate the entire surface of a Sabouraud dextrose agar plate. Swab plates were incubated for 72 hours in the control incubator and then assessed for growth or no growth. Five of the sampled books |