CELLULOID OBJECTS: THEIR CHEMISTRY AND PRESERVATIONJULIE A. REILLY
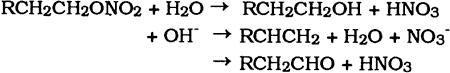
3 DEGRADATION3.1 INTRODUCTIONONCE A celluloid object has been fabricated, it becomes subject to the chemical, physical, and biological forces of its environment. The visual appearance of deteriorated celluloid When celluloid objects are fabricated with other materials such as metals, glass, textiles, and paper the problems of degradation are compounded. The deterioration products of celluloid are often corrosive (nitric acid) and react readily with other materials in proximity. As mentioned, celluloid's inherent degradation or aging process involves the recrystallization of cellulose nitrate molecules and the expulsion and sublimation of camphor. Environmental conditions generally alter this process by enhancing degradation of the cellulose nitrate molecules. Degradation through thermal, chemical, photochemical, and physical means generally leads to lower viscosities and softer rather than more brittle celluloid. Conservators must find the balance between these two processes if celluloid objects are to be preserved. 3.2 FORCES OF DEGRADATIONCelluloid deteriorates as a result of four degradation processes: thermal, chemical, photochemical, and physical. 3.2.1 Thermal DegradationThermal degradation of celluloid involves the breaking off of nitrate groups in the cellulose nitrate molecule. As a result, nitrous gases are evolved. The following reaction equation shows the probable mechanism whereby the weak -O-NO2 bonds are broken, leaving NO2 gas, aldehydes, and alcohols.
Selwitz indicates that nitrogen-oxygen bond cleavage takes place at temperatures above 100�C and when the molecule is exposed to visible and long-wave ultraviolet light (Selwitz 1988, 25). 3.2.2 Chemical DegradationChemical degradation can result from acid or alkaline hydrolysis. Acid hydrolysis involves the fission of glucosidic links in the cellulose nitrate molecule. With cellulose nitrate, this reaction is very slow and results in the reduction of the average molecular chain length of the cellulose nitrate molecule (Miles 1955, 268). Acids may be present as a result of synthesis, manufacture, or environmental conditions. Chemical breakdown of the cellulose nitrate molecule by alkalis is more rapid than that by acids—often called denitration—the following reactions show the results of alkaline degradation (after Miles 1955, 286).
Alkalis may also be present from synthesis, manufacture, and environment. Alkaline hydrolysis produces a wide variety of low molecular weight oxidized compounds. Inorganic nitrates, ammonia, cyanides, carbon dioxide, oxalic acid, maleic acid, glycolic acid, and malonic acid have all been recorded as deterioration products (Miles 1955, 278). Basic pigments added to cellulose nitrate lacquers were found to increase the rate of deterioration through nitrate loss in accelerated aging (Hercules 1955, 44–48). Acids may have been added to mixtures to neutralize alkaline pigments such as bone black, oil black, and nigrosine (Hercules 1955, 44–48). Aside from acid and alkaline hydrolysis, some metallic oxides are known to cause irreversible gelation of cellulose nitrate in solution (Chao 1934, 99–102). Lacquers, glues, and badly deteriorated celluloid can be irreversibly gelled by oxides of lead, calcium, arsenic, Selwitz points out that due to its structure the cellulose nitrate molecule is highly polar. This “extraordinarily high nonionic polarity” is also a contributing factor to the high instability of celluloid plastics (Selwitz 1988, 2). 3.2.3 Photochemical DeteriorationPhotochemical deterioration is relatively severe in celluloid due to the ability of the molecule to absorb strongly in certain wavelength ranges. The far ultraviolet is readily absorbed by celluloid. The maximum and complete absorbance occurs at around 2536 cm−1 (Miles 1955, 287). Different wavelengths of light have been found to deteriorate celluloid by reducing the viscosity and/or chain length of the molecules (Miles 1955, 291). The results of strong absorption can be seen as yellowing, embrittlement, and softening. One interesting breakdown mechanism apparently involves the disintegration of the nitrated glucoside ring structure by exposure to far-ultraviolet radiation (Selwitz 1988, 25). 3.2.4 Physical DegradationPhysical degradation of celluloid can be caused by thermal, chemical, and photochemical deterioration, loss of volatile constituents like camphor, and external physical stresses from fabrication or housings. Many objects appear to collapse on themselves as a result of the entrapment of harmful deterioration products in their interiors. The trapped gases accelerate deterioration in the immediate area and essentially consume the inside of the objects (Sirkis 1982). The thermal, chemical, photochemical, and physical breakdown of celluloid constitutes a set of highly interrelated decomposition reactions and mechanisms. Light, alkalis, acids, and certain metallic oxides can be very detrimental. The primary environmental contributor to celluloid degradation, however, appears to be moisture. Water is required for most of the aforementioned reactions. Water also has the potential to create physical stresses due to the isotropic nature of celluloid and its capacity for water absorption. Worden discusses a close relationship between nitrate content and absorption of water by cellulose nitrate (Worden 1911, 2:974). As the nitrate content drops (i.e., in degradation) cellulose nitrate can absorb more moisture from the atmosphere, increasing the rate of many water-dependent reactions (also see Miles 1955, 158–59) (Hercules, 1955, 36). This characteristic indicates that celluloid plastics may be more sensitive to moisture than are other more highly nitrated cellulose nitrate materials. |