ULTRAVIOLET-FLUORESCENCE MICROSCOPY OF PAINT CROSS SECTIONS:JOHN M. MESSINGER
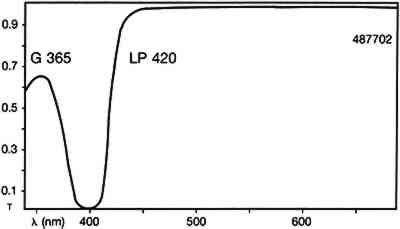
ABSTRACT—Cycloheptaamylose-dansyl chloride complex, a new fluorochrome specific for proteins, has been investigated as a stain to identify artists' paint media. Its selectivity and limitations are compared to those of the following commonly available fluorescent and nonfluorescent stains: lissamine rhodamine B sulfonyl chloride, 5-fluorescein isothiocyanate, amido black, Sudan black, rhodamine B, and 2′,7′-dichlorofluorescein. The methods used to examine the characteristics of these stains were reflected visible light microscopy and ultraviolet-fluorescence microscopy. The known samples of artists' media analyzed with the stains were linseed oil, casein, tempera, glair, gelatin, rabbit skin glue, and acrylic emulsions. 1 INTRODUCTIONThis study was undertaken to investigate the viability of using cycloheptaamylose-dansyl chloride complex (DC-C7A) as a fluorochrome marker for detecting proteins in paint cross sections. Dansyl chloride is a common reagent used for the fluorescent labeling and microanalysis of proteins (Kinoshita et al 1974, 1975). In water it is insoluble and hydrolyzed to the sulfonic acid. Dansyl chloride can be complexed with B-cyclodextrin to yield cycloheptaamylose-dansyl chloride complex, a reagent that is both soluble in aqueous urea solution, and very slow to hydrolyze. It was felt that this reagent might selectively stain proteins so that they could be examined by fluorescence microscopy. To ascertain the strengths and limitations of DC-C7A, it was imperative to compare the selectivity to stain protein containing materials and the color and uniformity of staining of DC-C7A to the those properties of two other fluorochromes commonly used to detect proteins in paint cross sections: lissamine rhodamine B sulfonyl chloride (LISSA) (Wolbers and Landrey 1987) and fluorescein isothiocyanate (FITC) (Wolbers and Landrey 1987; Wolbers 1988). These stains were also compared to the commonly utilized non-fluorochrome protein stain amido black (AB2) (Martin 1977), and the stains for oil: Sudan black (SB) (Johnson and Packard 1971), and the fluorochromes rhodamine B (RB) (Wolbers and Landrey 1987) and 2′,7′ dichlorofluorescein (DCF) (Wolbers and Landrey 1987). These stains were used to stain the following types of known media: linseed oil, casein, tempera, glair, gelatin, rabbit skin glue, and a few acrylic emulsions. Some of the media were also examined while loaded with such pigments as gypsum, whiting, titanium white, zinc white, turquoise, lamp black, and ultramarine. It was also noted whether the samples exhibited autofluorescence (fluorescence in the absence of any stain). 2 EXPERIMENTALThe various media were purchased commercially and painted onto glass microscope slides or After the known samples had cured, large representative chunks were removed from the test paintings and encased in cubes of Bio-Plastic polyester resin. After curing, the cross sections were ground and polished with odorless thinner as the cutting fluid. The samples were examined with a Zeiss Universal microscope with dark-field reflected light. The illumination was either in the visible light range with a 12 volt tungsten lamp or in the ultraviolet range with a high-pressure mercury vapor lamp. The filter set used with ultraviolet illumination was Zeiss catalog no 487702, which allows excitation of the sample with wavelengths up to 365nm and passes visible fluorescence above 420nm (fig. 1)
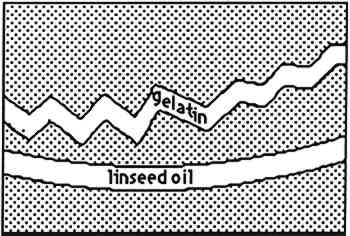
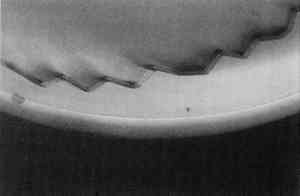
Photomicrographs were recorded on Kodak Ektachrome 200 daylight film (ED 135). When ultraviolet illumination was used, exposure times were four seconds. No filtration was used for ultraviolet illumination. To obtain proper color rendition with tungsten illumination, a Wratten 80A filter was required. DC-C7A is not commercially available, but its preparation from commercially available starting materials is easy. It was prepared, as outlined by Kinoshita, et al. (1975), by the following procedure: a 125 ml flask containing a magnetic spin bar was charged with 2.0 g of beta-cyclodextrin and 100 ml water. Fifteen minutes of stirring was required for complete dissolution of the cyclodextrin. Then 0.40 g of dansyl chloride was taken up in 3 ml anhydrous acetone and added to the cyclodextrin solution via dropper over 5 minutes. A fine yellow precipitate of the DC-C7A complex is formed in the flask during the course of addition. The flask and its contents were cooled in an ice bath for 30 minutes, centrifuged for 10 minutes, and the supernatant liquid decanted and discarded. The resultant lemon yellow powder was dried overnight over anhydrous CaSO4 at reduced pressure. The DC-C7A complex can be stored several months over CaSO4 in the dark. Just before use, about 10 mg of the DC-C7A complex is stirred into 5 ml of 8 M urea solution, and one drop of 10% triethanol amine in isopropanol (v/v) is added. The stain solution may be used immediately even though it takes 2–3 hours for all of the DC-C7A complex to dissolve. The solution remains effective as a stain for at least 4 hours. The stain was applied by soaking the sample cross sections in the solution for 8–10 minutes. The other fluorochromes were applied to the cross sections in solutions more dilute than those recommended by Wolbers and Landrey(1987), but otherwise the procedures were the same. It was felt that more dilute solutions would give The nonfluorochrome Sudan black (SB) was applied as recommended by Johnson and Packard (1971). A 60% ethanol:40% water (v/v) solution was saturated with SB. Cross sections were immersed in SB solution for about 30 minutes, then soaked in 40% ethanol:60% water (v/v) for 1 minute, then soaked in water 30 minutes. The amido black AB2 stain, as described by Martin (1977), was used exclusively: 100 mg of amido black was dissolved in 45 ml of glacial acetic acid to which is added 45 ml of 0.1 M aqueous sodium acetate followed by 10 ml of glycerine. Cross sections were immersed in the AB2 solution for approximately 5 minutes, rinsed with water, then soaked in 5% aqueous acetic acid for 5 minutes. 3 RESULTSTable 1 summarizes the staining results obtained from the samples examined and some of the results are illustrated in figures 2–13. Exact numbers of the number of staining attempts for each stain on each media, were not recorded. In fact, a particular stain was used to stain a particular media from one to 10 times because most the cross sections contained three or more known layers. With one exception, there was complete reproducibility of results when a particular stain was used to stain a particular layer. The exception, LISSA on pure linseed oil,sometimes yielded spotty pink blotches(fig. 7)while at other times it yielded a uniformly pink coloration of the media. The blotchy result seems to be related to the excavation of the surface of the sample. TABLE 1 STAINING RESULTS
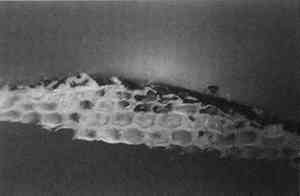
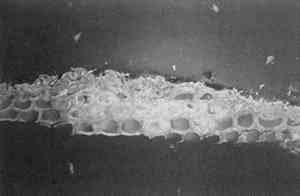
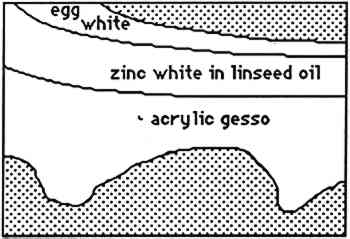
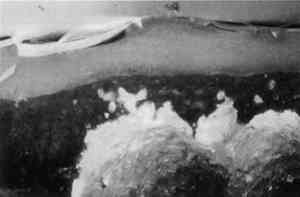
4 CONCLUSIONSThe results obtained with SB are fully consistent with that obtained by Johnson and Packard (1971). SB will stain layers containing acrylic emulsion a blue to black color. The results obtained with AB2 are fully consistent with those obtained by Martin (1977). It fails to stain acrylic emulsion layers to any significant degree. The results obtained with RB and DCF are fully consistent with those obtained by Wolbers (1987);. Layers containing acrylic emulsions are stained varying shades of red to yellow by RB and yellow-green to green by DCF. It has been pointed out that these results—using knowns—seem more consistent that the results typically found when stains are sued with unknowns, especially from older paintings (Gifford 1991). Both LISSA and FITC consistently yield a positive result when used to stain linseed oil containing certain pigments and with media based on acrylic emulsions. In fact, upon staining with FITC, a sample containing three layers: egg albumin over zinc white in oil, over acrylic gesso, left the albumin layer unstained, stained the zinc white layer bright green, and stained the acrylic gesso layer dim green. Similarly, LISSA consistently (tested three times with different lot numbers of the stain and delivery solvent) marked a sample very convincingly for protein. Yet this sample contained only linseed oil with whiting. It was observed that the acetone delivery solvent used with LISSA and FITC would etch the surface of the young linseed oil samples used in this study. This fact may explain some of the discrepancy between these findings and those of Wolbers (1987, 1988). However, it cannot be The fluorescent protein stains contain sulfonyl chlorides or isothiocyanates that are highly electrophilic functional groups. These materials react quickly and exothermically with nucleophiles such as hydroxyl groups (−OH), and in particular with primary amines (−NH2) and thiols (−SH) all of which are found in proteins. Sulfonyl chlorides react with water to form sulfonic acids and isothiocyanates react with water to form thiourethanes. One might conjecture that they would react with water that is adsorbed onto the surface of pigment particles and remain tightly bound to these particles in the same manner in which polar compounds are adsorbed onto the surface of the silica gel particles used in chromatography. It is interesting to note that manufacturers usually coat titanium oxide with silica and/or alumina to aid in its dispersion in the media and to improve the durability of the paint media (Whitehead 1978). The results of this study are consistent with the hypothesis that fluorochromes with electrophilic groups are reacting with water adsorved onto the surface of the polar micelles present in acrylic dispersion paints. DC-C7A is the only protein-selective, fluorochrome stain in this study to stain casein-based paint media (see figs. 2–4). Although it contains a sulfonyl chloride as its protein reactive functional group, it does not yield as many false positives with oil-containing layers as do LISSA and FITC. It uses an aqueous, alkaline delivery system that causes excavation of gelatin containing layers. However, this alkaline delivery system is advantageous in certain circumstances, as it did not excavate the carbonate- or oil-containing layers examined in this study. It does not stain iron oxide pigment in either linseed oil or Elvacite 2044. DC-C7A appears to be far more reliable than the other fluorochrome stains, as it does not stain most linseed oil-containing layers to any significant degree. It would be prudent to confirm the presence of titanium oxide before drawing any conclusions as to the presence of protein or acrylic media. DC-C7A does not stain egg white to any significant degree. ACKNOWLEDGEMENTSThe author wishes to acknowledge F. Christopher Tahk, Dan Kushel, and Christopher Augerson for their helpful suggestions and lively discussions. REFERENCESGifford, M.1991. Private communication. Johnson, M., and E.Packard. 1971. Methods used for identification of binding media in Italian paintings of the 15th and 16th centuries. Studies in Conservation16: 145–64. Kinoshita, T., F.Iinuma, and A.Tsuji. 1974. Flourescent labeling of proteins and a plasma membrane using cycloheptaamylose-dansyl chloride complex.Analytical Biochemistry61: 632–37. Kinoshita, T., F.Iinuma, and A.Tsuji. 1975. Microassay of proteins on membrane filter in the nanogram range using cycloheptaamylose-dansyl chloride complex.Analytical Biochemistry66: 104–9. Martin, E.1977. Some improvements in technique of analysis of paint media.Studies in Conservation22: 63–67. Whitehead, J.1978. Titanium compounds (inorganic). Kirk-Othmer Encyclopedia of Chemical Technology, 3d ed.New York: J. Wiley and Sons. 23:131–76.
Wolbers, R. C.. 1988. Aspects of the examination and cleaning of two portraits by Richard and William Wolbers, R. C., and G.Landrey. 1987. The use of direct reactive fluorescent dyes for the characterization of binding media in cross sectional examinations. AIC preprints, 15th Annual Meeting, American Institute for Conservation, Washington, D.C.168–202. AUTHOR INFORMATIONJOHN M. MESSINGER II received his Ph. D. in organic synthesis from the State University of New York at Buffalo in 1986. Since 1986 he has held the position of assistant professor of conservation science in the Art Conservation Department of State University College at Buffalo. Address: RH 230, State University College at Buffalo, Buffalo, NY 14222.
 Section Index Section Index |