PHYSICAL AND MECHANICAL PROPERTIES OF ALBUMEN PHOTOGRAPHSTIMOTHY VITALE, & PAUL MESSIER
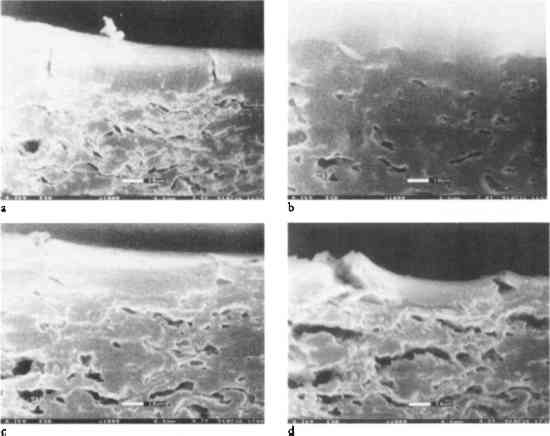
1 INTRODUCTIONIn a previous study (Messier and Vitale 1994), it was shown that all albumen prints exhibit cracking in the albumen layer. Aqueous treatment increases the number and width of these cracks and decreases the print gloss. In a study of the real-time behavior of albumen prints exposed to changes in relative humidity and water immersion using environmental electron scanning microscopy (E-SEM), similar behavior was observed (Messier and Vitale 1993). To explain this behavior, the evaluation of materials properties of albumen, an albumen and paper composite, and an experimental albumen print was undertaken. Figure 1 shows the dramatic effects of water on the albumen layer. This series of photo micrographs taken with an E-SEM shows a historical albumen print in cross section (Messier and Vitale 1993). The sample was placed in a grooved mount and not restrained in any way. The amount of water vapor (no other gas present) and the temperature of the sample were controlled within the E-SEM sample chamber. The sample was brought to 100% relative humidity and then immersed in water. Reversing the process, the sample was dried incrementally by decreasing the water vapor pressure in the sample chamber gradually to 14% relative humidity.
1.1 LITERATURE1.1.1 Egg Albumen
Messier (1991) reviewed the literature on the nature of egg albumen. The native albumen solution is a mixture of 13 globular proteins (Powrie and Nakai 1986). Of the six major polymeric proteins, ovalbumin is the largest constituent, 54% of native egg white. Egg white is approximately 88% water. Kinsella (1976) states that both mechanical denaturation and the introduction of salts will result in the availability of additional hydrophilic sites that were formerly within the protein globule. These sites increase the ability of the molecules to bind water. Bound water is attached through hydrogen bonds to polar side chains in lysine, glutamic acid, tyrosine, and similar amino acids, and to oxygen and nitrogen in the peptide bonds between individual amino acids (Bull 1944). In solution, the three-dimensional globular polymers have shells of tightly bound water surrounded by large quantities of associated water and massive quantities of bulk It is unclear if covalent cross-links exist in mechanically denatured egg albumen (Kuntz and Kauzmann 1974). Benesch and Benesch (1948), reviewing alcohol denaturation of egg albumen, report that denaturation seems to make sulfhydryl groups available. The primary Clovin (1964) and Putnam (1953) review the denaturation process. Putnam (1953) reports that egg albumen is denatured by the surface effects of frothing and that the egg albumen is the most susceptible of the proteins studied to denaturation by the action of coating. MacDonnell et al. (1950) show that mechanical shear (not pressure) results in denaturation, but that in solution the effects are partially reversed. Joly (1955) reports that mechanical denaturation can in some cases be partially reversed. Kauzmann and Simpson (1953) study the effects of chemical (urea) denaturation on the unfolding of the ovalbumin molecule due to disruption of hydrogen bonds. MacDonnell et al. (1955) studied the nature of single and multiple components of egg albumen that were both native and mechanically denatured. 1.1.2 Gelatin: A Related ProteinGelatin is a related globular protein. Bienkiewicz (1990) and von Endt and Baker (1991) review leather-water and gelatin systems. Harrington and Von Hippel (1961) review the structure of collagen and gelatin. Gelatin is a single helical molecule from the collagen triple helix. When separated in solution, gelatin takes a globular form. Literature on the effects of humidity changes on gelatin are of great value for interpreting the behavior of albumen. Calhoun and Leister (1959) studied the dimensional stability of an unsupported gelatin emulsion layer. They found that a unsupported Kodalith emulsion shrinks 0.7% between 70% and 80% RH, and they attribute this shrinkage to moisture-induced relaxation of dried-in strain. Mecklenburg (1991) reports that rabbit skin glue (gelatin) shrinks each time it is cycled to 95% after being taken to lower relative humidity. Karpowicz (1989) reports that rabbit skin glue, when dried with different types of restraint, will always shrink upon elevation to high humidity and return to normal humidity. He corroborates that glue continues to shrink when cycled (11 times) from high (83% and 91%) to moderate (54%) relative humidity. 1.1.3 Hardening of Albumen PrintsKlotz (1953) and Benesch and Benesch (1948) showed that silver strongly binds to functional groups, specifically sulfhydryl groups. Benesch and Benesch (1948) found that silver is complexed by crystalline egg albumen and cystine hydrochloride and suggested that denaturation makes more sulfhydryl groups available, the assumption being that cystine groups are the binding sites. Cystine is found in several protein polymers in egg albumen: ovalbumin, ovotransferrin (formerly conalbumin), ovo-mucoid, and lysozyme. However, in most of the protein present in albumen, the cystine is a complex of two amino acids joined with a disulfide bond. Free cystine is predominately available only in ovalbumin (one or two groups), which is the primary constituent (54%) of egg albumen.
MacDonnell et al. (1955) and Cunningham (1976) determined that ovalbumin was the major component of drainage from frothed egg white. Drainage could be as high as 60–75%, depending on the method of frothing; multiple frothings yielding the highest percentage of ovalbumin. Lysozyme, ovotransferrin, ovomucoid, and ovomucin were partially decreased by drainage from froth. Cunningham (1976) also found that globulins A1 and A2 were totally removed in the froth. When denatured, ovalbumin can have up to four available sulfhydryl groups (Feeney 1964); though Cunningham Ovotransferrin is known to act similar to blood proteins that sequester metal ions. Ovotransferrin contains only double cystine groups linked by a disulfide bond and no free sulfhydryl groups (Powrie and Nakai 1986). Alderton et al. (1946) has shown that ovotransferrin has the in vivo biological function of sequestering di- and trivalent metal ions that interfere with activity of the egg. Windle et al. (1963) states that there is strong evidence that propitiously spaced pendant oxygen, nitrogen, and bicarbonate (groups) bind metal ions in ovotransferrin. Munson (1993) has confirmed that in modern albumen print making, sensitized albumen is not soluble in the wash water, whereas the presensitized, salted, and denatured albumen is soluble. Towler (1864) remarks that sensitized albumen prints are coagulated (i.e., “set”) by sensitization and that they are quite hard and require no varnish. The introduction of silver into albumen-coated paper makes the sensitized material somewhat insoluble, but permeable, to water. Based upon the literature, it appears that silver is bound both to (1) oxygen, nitrogen, and other electron-rich pendant groups in ovotransferrin, and to (2) free sulfhydryl groups in ovalbumin and other protein polymers if opened by mechanical denaturation. It is probable that favorably spaced pendant groups between protein molecules also bind metal ions. Towler (1864) reports that unsalted albumen will sensitize weakly when exposed (floated) on a silver nitrate bath; this property indicates that some silver binding capability exists in mechanically denatured egg albumen. It is not known if disulfide bonds form between the protein polymers as egg albumen dries. If disulfide cross-links are created between polymer chains during drying, this result would satisfy the traditional explanation for “hardening” a material, i.e., making it less soluble or insoluble. That fact that dried-down, denatured, and salted albumen remains soluble in water suggests that few if any new disulfide bonds form as a result of drying. The behavior reported by both Munson (1993) and Towler (1864) suggests that the introduction of silver creates some form of “cross-links” that “hardens” egg albumen. Since drying egg albumen does not result in insolubility, then the binding effect described by Windle et al. (1963) for ovotransferrin is a strong candidate for the “hardening” of albumen because the appropriate pendant groups exist in the albumen-coated paper when it is exposed to silver during sensitization. |