GAS CHROMATOGRAPHIC ANALYSIS OF AMINO ACIDS AS ETHYL CHLOROFORMATE DERIVATIVES.MICHAEL R. SCHILLING, HERANT P. KHANJIAN, & LUIZ A. C. SOUZA
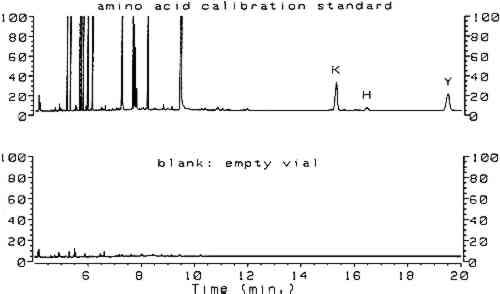
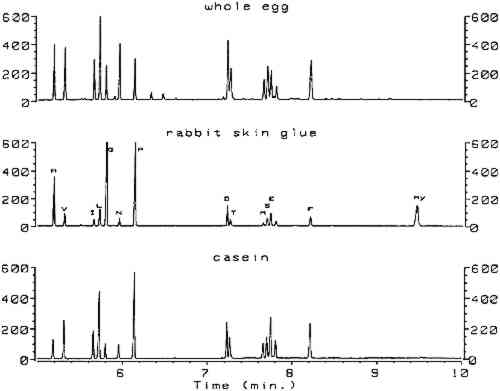
3 RESULTSComplete procedures for vapor-phase hydrolysis of proteins, derivatization of amino acids using ECF, and GC analysis with splitless injection and flame ionization detection (FID) are outlined in appendix 2. Information required to keep the procedure working reliably on a daily basis is described in appendix 3. Figure 1, an overlay of the analytical results for the amino acid calibration standard and a blank, shows that all the ECF-derivatized amino acids are resolved well. Although detection of histidine, tyrosine, and cysteine was possible, peaks for these amino acids do not exhibit the reproducibility required for proper quantitation. As stated earlier, because arginine does not derivatize by ECF, it cannot be detected. Figure 2 shows the chromatograms resulting from the analysis of whole egg, rabbit skin glue, and casein.
All protein amino acid compositions reported in tables 1, 2, and 3, including those obtained from the literature, are expressed in mole percent of only those amino acids detected by the ECF method. In addition, the weight percent of protein present in the samples could be estimated from the sum of the weight percentages of all ECF-derivatized amino acids detected. To correct for losses in the hydrolysis reaction, the weight percent of protein was TABLE 1 AMINO ACID COMPOSITION DATA FROM VARIOUS SOURCES FOR SELECTED PROTEINS TABLE 2 AMINO ACID COMPOSITION DATA FROM VARIOUS SOURCES FOR SELECTED EGG PROTEINS TABLE 3 AMINO ACID COMPOSITION DATA FROM VARIOUS SOURCES FOR SELECTED PROTEINS Table 1 summarizes the amino acid composition data for casein, glue, and gelatin samples. Data for egg proteins are listed in table 2. The results for blood, plant gums, other proteins, and proteinaceous contaminants are listed in table 3. In general, although the data listed in tables 1, 2, and 3 were acquired using several different analytical techniques, surprisingly good agreement was demonstrated. This fact illustrates that all the analytical techniques work well for pure, unpigmented samples of fresh protein. Interesting results were obtained from the analysis of common plant gums (see table 3). All gums tested contained low levels of amino acids. Furthermore, low norleucine internal standard recoveries were consistent with observations that condensation products can form between the carbohydrates and the free amino acids, reducing overall yields (White 1984). However, the most significant observation was that hydroxyproline was present in every gum tested, in concentrations of up to 29 mole percent for samples of gum arabic. |