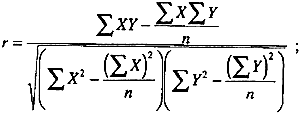GAS CHROMATOGRAPHIC ANALYSIS OF AMINO ACIDS AS ETHYL CHLOROFORMATE DERIVATIVES.MICHAEL R. SCHILLING, & HERANT P. KHANJIAN
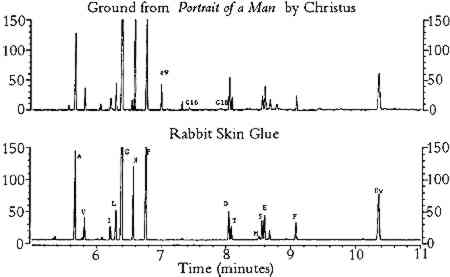
ABSTRACT—Proteinaceous binding media present in paintings can be identified by gas chromato-graphic analysis of the ethyl chloroformate derivatives of amino acids. The effects of pigments on the amino acid composition data of hydrolyzed gelatin that were obtained by this method were studied. The method was also used to analyze glue and egg tempera paint films that were exposed to heat and light in order to accelerate the aging of the paints. Pigments and accelerated aging were found to affect the concentrations of all amino acids, although the concentrations of alkyl and imino-substituted amino acids were affected to a much smaller degree than others. Samples of grounds and paints that were removed from a number of paintings and painted objects were analyzed by this method. The proteins present in the samples were identified from the amino acid molar percentages by correlation to published amino acid composition data. RESUM�—Les milieux agglom�rants prot�ineux pr�sents dans les tableaux peuvent �tre identifi�s � travers l'analyse chromatographique des d�riv�s d'�thyle chloroformat� des amino-acides. Les effets des pigments sur les donn�es de la composition des amino-acides de la g�latine hydrolis�e obtenues par cette m�thode ont �t� �tudi�s. Ce proc�d� a aussi �t� utilis� pour analyser les pellicules de peinture de tempera et de la colle et de l'oeuf expos�es � la chaleur et � la lumi�re afin d'acc�l�rer leur vieillissement. Les pigments et le vieillissement acceler� ont affect� la concentration de tous les amino-acides. Cependant, la concentration des alkyl amino-acides et des amino-acides d'imino remplacements ont �t� beaucoup moins affect�s. Les �chantillons des peintures et des fonds que l'on a pr�lev�s d'un certain nombre de tableaux et d'objets peints ont �t� analys�s selon cette m�thode. Les prot�ines pr�sentes dans les �chantillons ont �t� identifi�es � partir du pourcentage de la masse molaire des amino-acides par une corr�lation avec les donn�es publi�es sur la composition des amino-acides. RESUMEN-Los adhesivos prote�nicos que se pueden encontrar en algunos cuadros pueden ser identificados usando cromatografia de gases basada en los derivados de aminoacidos que se forman por reacci�n con el cloroformato de etilo. Estos estudios incluyeron los efectos de pigmentos sobre la composicion de aminoacidos resultantes de la hidrolisis de gelatina producida por el m�todo citado. El metodo fue usado tambien en el analisis de cola adhesiva y de pel�culas de pintura de tempera al huevo que fueron expuestos al calor y la luz, para acelerar el envejecimiento de las pinturas. Se encontro que los pigmentos y el envejecimiento acelerado afectan las concentraciones de todos los aminoacidos, aunque la concentracion de aminoacidos sustituidos con grupos alkilo o imino fue afectada en menor cantidad. El metodo se uso para analizar muestras de residuos solidos y pinturas obtenidos de un numero de pinturas y objetos pintados. La identificacion de las proteinas presentes en las muestras fue basada en los porcentajes molares de los aminoacidos encontrados usando correlacion a publicaciones anteriores sobre la composicion del aminoacidos. 1 INTRODUCTIONA number of chromatographic procedures have been developed for identification of proteins that are present in egg albumin, blood serum, and skin (Bidlingmeyer et al. 1984; Pickering However, it is not clear that the same analytical procedures would give reliable results for egg, casein, and animal glue tempera paints that contain inorganic pigments. Some inorganic compounds are known to interfere with the hydrolysis and derivatization reactions, thereby reducing the concentrations of some or all amino acid derivatives (Grzywacz 1994; Bidlingmeyer et al. 1984). The amount of reduction depends upon both the composition of the pigment present and the derivatization procedure. For example, high-performance liquid chromatography (HPLC) was used to measure the concentrations of amino acids (in the form of phenylthiocarbamyl derivatives) that were pres-ent in acid hydrolysates of glue and egg tempera paints (Halpine 1992). Copper pigments were found to reduce the concentration of all amino acid derivatives, whereas calcium pigments reduced the concentration of aspartic acid and glutamic acid derivatives only. In contrast, White (1984) used a gas chromatographic (GC) procedure (involving derivatization by trifluoroacylation and methylation) to analyze acid hydrolysates of the gypsum ground of a painting. It was found that the concentrations of aspartic acid and glutamic acid derivatives were unaffected by calcium interferences. From these observations it may be concluded that calcium interferes with the formation of phenylthiocarbamyl derivatives of aspartic acid and glutamic acid, not with the liberation of these amino acids from the proteins during acid hydrolysis. In certain instances, it may be possible to minimize pigment interferences by removing cations from amino acid hydrolysates prior to derivatization. In an HPLC (phenylthiocarbamyl derivatization) procedure used to analyze the proteins present in large samples of plaster (Ronca 1994), pigment interferences were greatly reduced by eliminating calcium from the amino acid hydrolysates using an ion-exchange resin. Aspartic acid and glutamic acid concentrations in acid hydrolysates of protein-containing plaster samples closely matched those of the reference samples of protein. Unfortunately, ion exchange may not be a feasible option for removing inorganic cations from acid hydrolysates of microsamples of paint, due to losses incurred by transfer steps and column holdup. Accordingly, it is important to study the extent to which pigments interfere with the results of any chromatographic procedure used for the identification of proteinaceous binding media. Physical aging is another factor that may complicate protein identification. It is well documented that proteins are susceptible to the effects of aging and that certain amino acids, such as cysteine and methionine, are more susceptible to chemical alteration and decomposition than others (Karpowicz 1981). However, the impact of physical aging on the chromatographic identification of proteins has yet to be established. Paint samples that contain two or more proteinaceous binding media may present particular problems to the analyst. In some instances samples of paint may contain more than one proteinaceous medium from the intentional mixing of media by the artist. More com-monly, samples of paint removed for analysis may be unavoidably contaminated with traces of underlying ground layers. The two proteinaceous media most likely to be present This paper is a continuation of the research reported in a previous article (Schilling et al. 1996) in which a gas chromatographic procedure was developed for analysis of unpigmented, unaged protein. The procedure was a modification of a method originally developed for blood serum analysis (Hušek 1991). One advantage of ethyl chloroformate (ECF) derivatization is that fatty acids, present as triglycerides in drying oils and other lipids, may be converted to ethyl esters and analyzed with the amino acid derivatives. This additional qualitative information may aid in differentiating egg yolk from egg white (Nowik 1995). The modified procedure employed vapor-phase acid hydrolysis of protein, derivatization of the free amino acids using ethyl chloroformate, splitless injection of the N(O,S)-ethoxycarbonyl amino acid ethyl ester derivatives, separation of the esters on an HP-INNOWAX capillary column, and flame ionization detection (FID). Amino acid compositions were reported in terms of moles per 100 moles detected, and the weight percent of protein was estimated from the sum of the weight percentages of all amino acids detected. Sample weights were determined to the nearest 0.1 mg on an ultramicrobalance. Henceforth, this procedure will be referred to as the ECF procedure. A list of pertinent information regarding amino acids appears in appendix 1. 2 SCOPE OF RESEARCH2.1 PIGMENT INTERFERENCES IN ECF PROCEDUREA study was conducted to identify the effects of pigments in the ECF procedure. Gelatin was hydrolyzed with samples of 22 different artists' pigments that were selected on the basis of chemical composition to include most of the common pigments. Samples were prepared using the following procedure. Approximately 100 μg of dry pigment was weighed onto an aluminum pan and transferred to a 1 ml hydrolysis tube, to which was added 100 μl of an aqueous solution of food-grade gelatin (approximately 1 μg of gelatin/μl) and 2 μl of L-norleucine internal standard (1500 μg /μl in 0.1M HCl). After the solution was evaporated to dryness with a stream of nitrogen, the tubes (a maximum of 13) were placed inside a 25 ml hydrolysis chamber. Included with each set of samples were an empty tube that served as a blank and another tube that contained 100 μg of rabbit skin glue to monitor the hydrolysis yield. Half the hydrolysate solution was derivatized, following the ECF procedure (Schilling et al. 1996). To dissolve the derivatives prior to injection, 200 μl of benzene was used. Due to the toxicity of the reagents, a fume hood should be employed during the derivatization step. The concentrations of amino acids in the pigmented gelatin hydrolysates were compared to those of unpigmented gelatin, and the amount of protein in the sample was estimated from the sum of the weight percentages of all ECF-derivatized amino acids. 2.2 EFFECTS OF ACCELERATED AGING ON AMINO ACID COMPOSITION OF TEMPERA PAINTTo ascertain the effects of light and heat aging on proteinaceous paint media, egg yolk paints and rabbit skin glue paints were prepared with selected pigments and known pigment-to-binder composition following traditional procedures (Kay 1983), applied to glass plates, and allowed to dry at room temperature for 9 months. Afterward, one set of all paint samples was aged at 80�C for 6 weeks; an identical set was placed in a Weather-o-meter exposure chamber at 50�C and 50% RH for 500 hours; and a third set was stored at ambient conditions After aging, the paints were sampled for GC analysis. Sample weights were <100 μg for egg paints and <300 μg for glue paints. Half the hydrolysate solution was derivatized, and benzene was used to dissolve the derivatives (from 50 μl to 200 μl was used, depending on the amino acid concentration). The amino acid compositions of the aged paints were compared to those of paints stored in ambient conditions. Changes in amino acid concentration were calculated for each paint mixture, and estimates of total protein content were compared to data that were derived from proximate analysis (Bergquist 1981). 2.3 SCHEME FOR IDENTIFICATION OF PROTEINACEOUS BINDING MEDIAA scheme for protein identification was developed based on the molar compositions of the amino acids that were essentially unaffected by pigment interferences and aging. The scheme incorporated data analysis techniques employed in other studies: concentration tables (Halpine 1992; Ronca 1994) and correlation matrices, discussed in appendix 2(Sinkai and Sugisita 1990). In addition, estimates of protein content were useful for eliminating erroneous matches from the correlation matrix results (see data for Mantegna's Holy Family, listed in section 3.3). Reference data used in the identification scheme were taken from the compilation of amino acid compositions reported in Schilling et al. 1996. Multiple sets of composition data for collagen (rabbit skin glue, fish glue, collagen), casein, and egg were reduced to averages for simplicity. The data listed in appendices 3 and 4 were expressed in molar percentage formats consistent with the protein identification scheme. To more readily identify mixtures of whole egg and rabbit skin glue that may be present in objects, paints consisting of mixtures of these media were prepared. Lead white was ground with whole egg and rabbit skin glue mixed in varying proportions. The paints were applied to glass plates and allowed to dry under ambient lighting, temperature, and relative humidity conditions for four years. Samples were removed for GC analysis, and the concentration data were evaluated using the protein identification scheme. Experimentally determined protein content data were compared to the known composition of the paints to assess the accuracy. 2.4 ANALYSIS OF PAINT SAMPLES REMOVED FROM OBJECTSTo illustrate the utility of the ECF procedure, paint and ground samples from a number of objects were analyzed. From the analytical results, protein content was estimated and the proteinaceous binding media were identified. For most samples, the entire hydrolysate volume was derivatized, and the derivatives were dissolved in 10 μl of benzene prior to injection. Sample weights ranged from 6 μg to 40 μg for most samples; larger samples were analyzed when appropriate, based on the expected protein content. 3 RESULTS3.1 EFFECTS OF PIGMENTS ON AMINO ACID COMPOSITIONThe results from the study of the effects of pigments on the amino acid composition of hydrolyzed gelatin are listed in tables 1 and 2. As shown in table 1, pigments reduced the concentration of amino acids that possess certain functional groups: acidic (aspartic acid, glutamic acid); hydroxyl (threonine, serine); basic (lysine); sulfur-containing (methionine); and aromatic (phenylalanine). For simplicity, these amino acids will be referred to as “reactive.” Aspartic acid, glutamic acid, serine, and threonine were especially sensitive to hematite, ochres, umber, azurite, and malachite. The TABLE 1. COMPOSITION OF GELATIN HYDROLYZED IN THE PRESENCE OF SELECTED PIGMENTS TABLE 2. MOLE PERCENTAGE COMPOSITION OF GELATIN HYDROLYZED IN THE PRESENCE OF SELECTED PIGMENTS The concentrations of amino acids substituted with alkyl groups (alanine, valine, isoleucine, leucine, glycine) and imino groups (proline, hydroxyproline) were less affected by pigment interferences; these amino acids will be referred to as “stable.” Variation in ppm concentrations (as measured by the percent relative standard deviation for all pigmented gelatin samples) was approximately 15% for stable amino acids and 30% for reactive amino acids. A few pigments (red lead, verdigris, and orpiment) greatly reduced the concentration of the internal standard, norleucine. Because all amino acid concentrations were normalized to the recovery of norleucine, these pigments caused anomalous increases in ppm concentration for all amino acids (except methionine) and the total protein content of the gelatin solution. The molar composition data (moles per 100 moles of ECF-derivatized amino acids) for the pigmented gelatin solutions are listed on the left side of table 2. Relative mole percent data are less affected by pigment interferences than are the ppm concentration data: the relative standard deviation of the mole percent data for stable amino acids is approximately 6% (versus 13% for the ppm data), and it is 40% for reactive amino acids (versus 46% for the ppm data). For a given amino acid, relative molar composition data exhibit lower relative standard deviations because the concentrations of most amino acids are reduced by pigments. Listed on the right side of table 2 are the stable amino acid molar percentages (moles of stable amino acid per 100 moles of stable amino acids detected). It is apparent that stable amino acid mole percentages are less sensitive to pigment interferences than are the composition data normalized to the sum of all amino acids detected. For example, the stable amino acid composition data for gelatin hydrolyzed in the presence of hematite, burnt umber, or Prussian blue are nearly equal to the composition of unpigmented gelatin, whereas the corresponding data on the left side of table 2 are widely divergent. For most pigments, the changes in stable amino acid molar percentages are less than 10% with respect to unpigmented gelatin. This finding indicates that proteins present in unaged paint may be identified from amino acid molar percentage data derived from the ECF procedure without risk of serious pigment interferences. 3.2 EFFECTS OF ACCELERATED AGING ON AMINO ACID COMPOSITION OF TEMPERA PAINTTables 3 and 4 list the results from the study of the effects of accelerated aging on rabbit skin glue and egg yolk paints, respectively. It should be noted that, as a result of a peak overlap of threonine with oleic acid ethyl ester, the data for threonine in egg yolk paints are inaccurate. However, the concentration of oleic acid is greatly reduced with light or thermal aging; hence the threonine data for the aged paints are closer to the expected values. Oleic acid may be extracted from the hydrolysate prior to derivatization by treatment with chloroform (Schilling et al. 1996), but this step was not performed in order to simplify the derivatization procedure. As mentioned earlier, the data for methionine exhibit greater variability due to the absence of 2-mercaptoethanol stabilizer during hydrolysis. TABLE 3. AMINO ACID WEIGHT PERCENT COMPOSITION OF AGED RABBIT SKIN GLUE TEMPERA PAINTS TABLE 4. AMINO ACID WEIGHT PERCENT COMPOSITION OF AGED EGG YOLK TEMPERA PAINTS The results in tables 3 and 4 indicate that aging had a small effect on the total protein content of animal glue paints, whereas the protein content of egg yolk paints decreased substantially after aging (especially paints that were exposed in the Weather-o-meter). Aging caused a 25% reduction in protein content (the total weight percentage of all amino acids detected) relative to paint stored under ambient conditions. This result is due in part to the fact that egg contains a smaller weight percentage The amino acid profiles for rabbit skin glue paints matched unpigmented, unaged glue with a correlation coefficient greater than 0.994, indicating a high degree of similarity. In contrast, the correlation coefficients between the amino acid composition data of aged egg yolk tempera paints and unaged, unpigmented egg yolk were as low as 0.78, indicating that considerable changes in composition had occurred during aging. In general, egg yolk paints were more affected by heat and light than were glue paints, regardless of the pigment present. Light-aging produced the most extreme changes in amino acid concentration for both media. Table 5 lists the stable amino acid percentage data for the aged paints. Relative standard deviations for the set of data averaged 9% for all amino acids. Correlation coefficients comparing the data for the aged paints to unaged, unpigmented media were greater than 0.93 for all paint samples. The results presented in table 5 show that stable amino acid molar percentages are much less affected by the effects of aging than are data normalized to the yield of all detectable amino acids. TABLE 5. STABLE AMINO ACID MODEL PERCENT COMPOSITION OF AGED PAINT FILMS 3.3 ANALYSIS OF SAMPLES FROM OBJECTS OF ARTListed in tables 6, 7, and 8 are the data from the analysis of samples of paint and ground removed from art objects and the paints made from lead white mixed with rabbit skin glue and whole egg. The molar percentage data for all amino acids detected are listed in table 6. Stable amino acid composition data are listed in table 7. Correlation coefficient data that indicate the degree of similarity between the stable amino acid composition data of the objects with the data for selected proteinaceous reference materials are presented in table 8. The data presented in the tables for proteinaceous reference materials were selected from the listings in appendices 3 and 4. Coefficients of correlation were calculated between the object data and the data for each of the materials listed in appendix 4. For simplicity, only those materials that gave correlation coefficients greater than 0.9 were included in tables 6, 7, and 8. TABLE 6. COMPOSITION OF SAMPLES FROM OBJECTS AND REFERENCE MATERIALS, AS DETERMINED BY GAS CHROMATOGRAPHIC ANALYSIS OF AMINO ACID-ETHYL CHLOROFORMATE DERIVATIVES TABLE 7. COMPOSITION OF SAMPLES FROM OBJECTS AND REFERENCE MATERIALS, AS DETERMINED BY GAS CHROMATOGRAPHIC ANALYSIS OF AMINO ACID-ETHYL CHLOROFORMATE DERIVATIVES TABLE 8. CORRELATION COEFFIECIENTS FOR SAMPLES FROM OBJECTS AND REFERENCE MATERIALS, BASED ON STABLE AMINO ACID COMPOSITION The first example is a sample of ground from Portrait of a Man (late 15th century), a panel painting by Petrus Christus from the collection of the Los Angeles County Museum of Art (accession number 44.2.3). The painting was the subject of an in-depth technical analysis that was described in a presentation at a symposium on Petrus Christus, held in 1994 at the Metropolitan Museum, New York (Fronek 1994). The results of GC analysis showed the amino acid composition of the ground is typical for collagen (tables 6 and 7). The correlation coefficient of 0.998 (table 8) with respect to the data for collagen indicates the sample was not contaminated with egg tempera from the paint layers to any appreciable degree. It is not possible to identify the source of the collagen (such as rabbit skin glue, fish glue, or gelatin) based on the amino acid data. Extraneous peaks in the chromatogram (fig. 1), associated with fatty acids originating from lipids (Nowik 1995; Schilling et al. 1996), indicate that oil was also present in the sample. From an examination of paint cross sections, it was concluded that the oil was present in the imprimatura layer (Fronek 1994).
In a comprehensive study of the distemper paintings of Andrea Mantegna (Rothe 1992), medium identification, helpful in understanding the artist's technique, was undertaken using low-power microscopic examination, solubility tests, and various analytical techniques. Paint and ground samples removed from a large number of distemper paintings were used as study materials. The study resolved some of the common misconceptions about the media A sample consisting of brown paint removed from Mantegna's St. Mark (1448–49, St�delsches Kunstinstitut, Frankfurt) was found to contain high concentrations of alanine, hydroxyproline, and proline. The correlation coefficient data confirm the identity of the medium as collagen (table 8), although it is not clear why the glycine content is so much lower than expected. The result was in agreement with recent findings, based on solubility tests, which concluded that the medium was not egg yolk (Rothe 1992). GC analysis was performed on a sample of green paint from another Mantegna painting, Holy Family with St. Mary Magdalene (1495– 1505, catalog number 55 in the Altman Collection of the Metropolitan Museum of Art). No hydroxyproline was detected in the green (tables 6 and 7), thereby eliminating collagen as a possible medium (and also other materials that contain hydroxyproline). The correlation coefficient data (table 8) indicated the amino acid composition of the medium correlated closely to the composition of the amino acids in egg (and also the absurd materials rice and moth). The high protein content of the paint confirmed the presence of egg in the sample (it greatly exceeded that of rice). The ECF results for Holy Family with St. Mary Magdalene were inconsistent with earlier studies stating that egg is not present (Stulik 1991). This conclusion was based primarily on gas chromatography–mass spectrometry (GC-MS) results, which showed the absence of detectable quantities of cholesterol in a sample containing green and brown paint. However, the proportion of egg in the sample may have been much lower than anticipated due to the The ECF procedure was used to analyze a sample of red paint from the wall paintings of the tomb of Nefertari, located in the Valley of the Queens in Luxor, Egypt. Using various techniques, primarily carbohydrate analysis, the medium was identified in an earlier study as a type of gum arabic that was (and is still, today) available in and around Luxor (Stulik et al. 1993). This identification was supported by the amino acid composition data obtained from the ECF procedure (tables 6–8). The correlation coefficient data showed close correlation to either gum guar, gum arabic from Luxor, or lead white paint made with 5% rabbit skin glue mixed with whole egg; the hydroxyproline content of the paint is nearest to that of gum arabic from Luxor. As mentioned previously (Schilling et al. 1996), significant amounts of hydroxyproline are present in many plant gums, which may cause them to be mistakenly identified in paint samples as animal glue or gelatin. The final examples illustrating the utility of the ECF procedure are samples of ground and paint taken from painted altarpieces in Minas Gerais, Brazil (Souza et al. 1992). According to old recipes the most probable medium in the ground was parchment glue. Of particular importance is the fact that Fourier transform infrared spectrometry (FTIR) could not detect protein in any of the samples, indicating that the protein content of the grounds was below the FTIR detection limit of approximately 10% (Souza 1993). The protein in a ground sample from one of the altarpieces (CA134) correlates well to collagen (tables 6 and 7). The composition of a paint sample (CA046) comprised of particles of ground mixed with green and black paint is consistent with admixtures of collagen and egg. The correlation coefficient data indicate that the amino acid composition does not match that of any single proteinaceous medium (table 8). However, the correlation coefficient and ratio data match the results for the 2% glue/98% whole egg paint test mixture extremely well, suggesting that a mixture of the two media may indeed be present. This finding illustrates that the mixture data may aid in the identification of protein in paint samples from objects of art. 4 DISCUSSIONIt has been demonstrated that amino acids that possess reactive functional groups (acidic, basic, sulfur-containing, hydroxyl) are more affected by pigment interferences (in the ECF procedure) and exposure to heat and light than are the alkyl and imino-substituted amino acids. Thus, in schemes for identification of proteins that are based on absolute or relative concentrations of amino acids (Keck and Peters 1969; White 1984; Pancella and Bart 1989; Halpine 1992; Grzywacz 1994; Ronca 1994), greater emphasis should be placed on those amino acids with more stable functional groups than on those with more reactive groups. With respect to the concentrations of amino acids in acid hydrolysates, glue films tended to be more stable than egg yolk under identical conditions of heat and light exposure. This difference is due in part to the fact that glue has a high content of stable amino acids and correspondingly lower levels of easily photooxidized amino acids (such as histidine, tyrosine, and the sulfur-containing amino acids methionine and cysteine). In contrast, the chemical composition of egg yolk promotes photo-oxidation and radical-induced decomposition (Karpowicz 1981; Davies et al. 1987). Egg yolk has a much lower proportion of stable amino acids and higher concentrations of reactive amino acids (methionine and cysteine) that cause egg yolk films to be more susceptible to photo-oxidation. In addition, photo-induced Hydrolysates of light-aged glue paint films contained higher concentrations of isoleucine than the corresponding unexposed paints. A possible explanation for this result is that clusters of bulky alkyl groups, such as isoleucine, present a steric hindrance to hydrolysis, resulting in reduced isoleucine yields from hydrolysates of fresh, unaged proteins (Pellett 1981; Pickering and Newton 1990). Aging causes denaturing and partial disruption of the protein structure (Karpowicz 1981), exposing the alkyl group clusters to the action of aqueous acid, thereby causing improved hydrolysis yields. Another explanation for the higher concentration of isoleucine in aged samples is that a species formed during aging may coelute with isoleucine. GC-MS could be used to test this hypothesis but was not employed in this study. Regarding the estimation of protein content in test paint samples, the GC results agreed reasonably well (to within � 25%) with theoretical estimates based on elemental analysis (Bergquist 1981) and the known compositions of the paint test samples. Certain factors contributed to the success of the protein content estimates, such as minimizing the number of sample transfer steps and correction of hydrolysis yields through the use of a rabbit skin glue reference standard. In addition, the availability of an ultramicrobalance permitted micro-samples of paint to be weighed with high accuracy. These data should, however, be considered only as approximations, because many factors can have unforseen effects on the weight percent results (such as long-term aging effects, organic matrix effects, and pigment interferences). In general, the samples from objects contained somewhat less protein than the test paints. Aging certainly is responsible for loss of amino acids in the samples from objects. Also, in removing paint samples from objects, there is always the likelihood that particles may be inadvertently extracted from other layers that contain little or no protein but that contribute to the total sample weight, such as varnish and wax relining adhesive. Nevertheless, the estimates of protein content in paint from objects of art were much lower than the ion chromatography results reported by Keck and Peters (1969). In this article the average protein content was reported to be 45% for animal glue–based paint and 20% for egg yolk tempera paint. These data have been cited in other studies as typical protein contents for tempera paint and were used to estimate sample weights in HPLC studies (Halpine 1992). However, from an inspection of other results reported in this article, there may be adequate reason to suspect the accuracy of these data. For example, a sample of egg yolk tempera paint was found to contain 35% protein. However, unpigmented dried egg yolk contains less than 32% protein, as determined by elemental analysis (Bergquist 1981). In another example, it was reported that a sample of ground from the 14th century contained 92% protein, a percentage so high as to indicate that the ground is nearly pure glue with almost no gypsum, a composition that it is extremely unlikely. No explanation for these anomalous results was provided. 5 CONCLUSIONSThe results of the research reported here illustrate that proteins present as binding media may be identified reliably by gas chromatographic analysis of ethyl chloroformate derivatives of amino acids. Alkyl and imino-substituted amino acids were much less affected by pigments and aging than the other amino acids; hence, in the ECF procedure, protein identification should be based on the relative amounts of these amino acids only. Amino acid molar percentages were used, in conjunction with coefficients of correlation to published composition data, to identify the proteins ACKNOWLEDGEMENTSThe authors wish to thank the following members of the Scientific Program of the Getty Conservation Institute (GCI): Dusan Stulik, head, Analytical Section, who encouraged this research; Michele Derrick, former associate scientist, who provided invaluable advice on the Mantegna study; and James Druzik, William Ginell, David Scott, and Charles Selwitz, who reviewed this manuscript. Special thanks go to Andrea Rothe, head, paintings conservation, J. Paul Getty Museum, who was instrumental in obtaining samples from the Mantegna paintings and who provided extremely valuable advice and encouragement; Joseph Fronek, head, paintings conservation, Los Angeles County Museum of Art, who provided samples of the Petrus Christus and who, together with Shelley Svoboda, assistant paintings conservator, and John Twilley, senior research scientist, Los Angeles County Museum of Art, gave invaluable insight into the painting technique of Christus; Luiz Souza, a former research fellow at GCI and presently at CECOR, EBA, Universidade Federal de Minas Gerais, Belo Horizonte, MG, Brazil, who conducted the analyses of the Brazilian altarpiece samples; the Brazilian Institute of Cultural Properties; Hubert von Sonnenburg, head, paintings conservation, Metropolitan Museum of Art, New York; and Peter Waldeis, conservator, the St�delsches Kunstinstitut, Frankfurt. APPENDIX1 APPENDIX 11.1 AMINO ACIDS THAT MAY BE FOUND IN ACID HYDROLYSATES OF PROTEINS
2 APPENDIX 22.1 CORRELATION COEFFICIENTSA useful statistical tool for determining the degree of association between data sets is the correlation coefficient (Anderson 1987). It is defined by the following equation:
Sinkai and Sugisita (1990) used correlation coefficients to identify proteins in adhesives, by comparing concentration data for adhesives samples to data for proteinaceous reference materials and materials that contain amino acids, such as rice starch, glue casein, egg yolk, and gelatin. The method was quite successful and provided an easy means for comparing large data sets. 3 APPENDIX 33.1 AMINO ACID COMPOSITION DATA FOR VARIOUS PROTEINS
4 APPENDIX 44.1 STABLE AMINO ACID COMPOSITION DATA FOR VARIOUS PROTEINS
REFERENCESAnderson, R. L.1987. Practical statistics for analytical chemists. New York: Van Nostrand Reinhold. Bergquist, D.1981. Eggs. In Kirk-Othmer encyclopedia of chemical technology, 3d ed.New York: John Wiley and Sons. 8:429–45. Bidlingmeyer, B. A., S. A.Cohen, and T. L.Tarvin. 1984. Rapid analysis of amino acids using precolumn derivatization. Journal of Chromatography336:93–104. Davies, K.J.A., M. E.Delsignore, and S. W.Lin. 1987. Protein damage and degradation by oxygen radicals. Journal of Biological Chemistry262(20):9902–7. Fronek, J.1994. The Los Angeles Portrait of a Man. Paper presented at the symposium “The Petrus Christus in Renaissance Bruges,” Metropolitan Museum of Art, New York. Grzywacz, C. M.1994. Identification of proteinaceous binding media in paintings by amino acid analysis using 9-fluorenylmethyl chloroformate derivatization and reversed-phase high-performance liquid chromatography. Journal of Chromatography A676(1):177–83. Halpine, S.1992. Amino acid analysis of proteinaceous media from Cosimo Tura's The Annunciation with Saint Francis and Saint Louis of Toulouse. Studies in Conservation37:22–38. Haynes, P. A., D.Sheumack, J.Kibby, and J. W.Redmond. 1991. Amino acid analysis using derivatization with 9-fluorenylmethyl chloroformate and reversed-phase HPLC. Journal of Chromatography540:177–85. Hušek, P.1991. Rapid derivatization and gas chromatographic determination of amino acids. Journal of Chromatography552:289–99. Karpowicz, A.1981. Ageing and deterioration of proteinaceous media. Studies in Conservation26:153–60. Kay, R.1983. The painter's guide to studio methods and materials. Englewood Cliffs, N.J.: Prentice-Hall. Keck, S., and T.PetersJr.1969. Identification of protein-containing paint media by quantitative amino acid analysis. Studies in Conservation14:75–82. Nowik, W.1995. Acides amines et acides gras sur un meme chromatogramme-un autre regard sur l'analyse des liants en peinture. Studies in Conservation40:120–26. Pancella, R., and R.Bart. 1989. Identification des liants organiques dans les couches picturales par chromatographie en phase gazeuse. Zeitschrift fur Kunsttechnologie3(3):101–11. Pellett, P. L.1981. Proteins. In Kirk-Othmer encyclopedia of chemical technology, 3d ed.New York: John Wiley and Sons. 15:314–41. Pickering, M. V.1989. Ion-exchange chromatography of free amino acids. LC/GC7(6):484–90. Pickering, M. V., and P.Newton. 1990. Amino acid hydrolysis: Old problems, new solutions. LC/GC8(10):778–81. Ronca, F.1994. Protein determination in polychromed stone sculptures, stuccoes and gesso grounds. Studies in Conservation39:107–20. Rothe, A.1992. Mantegna's paintings in distemper. In Andrea Mantegna, ed.J.Martineau. London: Royal Academy of Arts. 80–88. Schilling, M., H.Khanjian, and L. A. C.Souza. 1996. Gas chromatographic analysis of amino acids as ethyl chloroformate derivatives. Part 1, Composition of proteins associated with art objects and monuments. Journal of the American Institute for Conservation35:45–59.
Sinkai, T., and R.Sugisita. 1990. Identification of Souza, L.A.C.1993. Personal communication. CECOR, EBA, Universidade Federal de Minas Gerais, Belo Horizonte, MG, Brazil. Souza, L.A.C., A. R.Ramos, and C.Avila. 1992. The matriz of Catas Altas, Minas Gerais, Brazil: Techniques, materials and style. Preprints of the Contributions to the Madrid Congress, ed.H.W.M.Hodges et al. London: International Institute for Conservation of Historic and Artistic Works. 154–57. Stulik, D. C.1991. Analysis report on samples from paintings by Andrea Mantegna. Getty Conservation Institute, Marina del Rey, Calif. August 14. Stulik, D. C., E.Porta, and A.Palet. 1993. Analyses of pigments, binding media, and varnishes. In Art and Eternity: The Nefertari wall paintings conservation project 1986–92, ed.M. A.Corzo and M.Afshar. Singapore: J. Paul Getty Trust. 55–65. White, R.1984. The characterization of proteinaceous binders in art objects. National Gallery Technical Bulletin8:5–14. SUPPLIERSPigments obtained from Kremer Pigments Inc., 61 East Third St., New York, N.Y. 10003 Names and addresses of other suppliers of materials and equipment are listed in Schilling et al. 1996. AUTHOR INFORMATIONMICHAEL R. SCHILLING earned his B. S. (1983) and M.S. (1990) in chemistry from the California State Polytechnic University, Pomona. He has worked at the Getty Conservation Institute since 1983 and presently holds the position of associate scientist. His interests are thermoanalytic methods, light microscopy, gas chromatography, mass spectrometry, and color measurement. He has been active in the examination of painted museum objects, pigment identification, binding medium analysis, and analysis of volatile organic compounds in the museum environment. He has also been involved in a number of special GCI collaborative projects: conservation of the wall paintings in the tomb of Nefertari, located in Luxor, Egypt; preservation of the wall paintings and sculptures in the Dunhuang and Datong grottoes, China; and conservation of the Dead Sea scrolls in Jerusalem, Israel. Address: Getty Conservation Institute, Scientific Program, 4503 Glencoe Ave., Marina del Rey, Calif. 90292. HERANT P. KHANJIAN received his B.A. degree in chemistry from California State University, Northridge. His research interests involve the detection and identification of binding media found in art objects using gas chromatography and infrared spectroscopy. Address as for Schilling.
 Section Index Section Index |