GAS CHROMATOGRAPHIC ANALYSIS OF AMINO ACIDS AS ETHYL CHLOROFORMATE DERIVATIVES.MICHAEL R. SCHILLING, & HERANT P. KHANJIAN
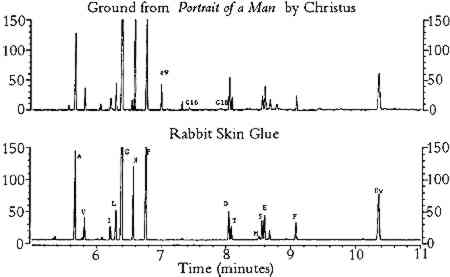
3 RESULTS3.1 EFFECTS OF PIGMENTS ON AMINO ACID COMPOSITIONThe results from the study of the effects of pigments on the amino acid composition of hydrolyzed gelatin are listed in tables 1 and 2. As shown in table 1, pigments reduced the concentration of amino acids that possess certain functional groups: acidic (aspartic acid, glutamic acid); hydroxyl (threonine, serine); basic (lysine); sulfur-containing (methionine); and aromatic (phenylalanine). For simplicity, these amino acids will be referred to as “reactive.” Aspartic acid, glutamic acid, serine, and threonine were especially sensitive to hematite, ochres, umber, azurite, and malachite. The TABLE 1. COMPOSITION OF GELATIN HYDROLYZED IN THE PRESENCE OF SELECTED PIGMENTS TABLE 2. MOLE PERCENTAGE COMPOSITION OF GELATIN HYDROLYZED IN THE PRESENCE OF SELECTED PIGMENTS The concentrations of amino acids substituted with alkyl groups (alanine, valine, isoleucine, leucine, glycine) and imino groups (proline, hydroxyproline) were less affected by pigment interferences; these amino acids will be referred to as “stable.” Variation in ppm concentrations (as measured by the percent relative standard deviation for all pigmented gelatin samples) was approximately 15% for stable amino acids and 30% for reactive amino acids. A few pigments (red lead, verdigris, and orpiment) greatly reduced the concentration of the internal standard, norleucine. Because all amino acid concentrations were normalized to the recovery of norleucine, these pigments caused anomalous increases in ppm concentration for all amino acids (except methionine) and the total protein content of the gelatin solution. The molar composition data (moles per 100 moles of ECF-derivatized amino acids) for the pigmented gelatin solutions are listed on the left side of table 2. Relative mole percent data are less affected by pigment interferences than are the ppm concentration data: the relative standard deviation of the mole percent data for stable amino acids is approximately 6% (versus 13% for the ppm data), and it is 40% for reactive amino acids (versus 46% for the ppm data). For a given amino acid, relative molar composition data exhibit lower relative standard deviations because the concentrations of most amino acids are reduced by pigments. Listed on the right side of table 2 are the stable amino acid molar percentages (moles of stable amino acid per 100 moles of stable amino acids detected). It is apparent that stable amino acid mole percentages are less sensitive to pigment interferences than are the composition data normalized to the sum of all amino acids detected. For example, the stable amino acid composition data for gelatin hydrolyzed in the presence of hematite, burnt umber, or Prussian blue are nearly equal to the composition of unpigmented gelatin, whereas the corresponding data on the left side of table 2 are widely divergent. For most pigments, the changes in stable amino acid molar percentages are less than 10% with respect to unpigmented gelatin. This finding indicates that proteins present in unaged paint may be identified from amino acid molar percentage data derived from the ECF procedure without risk of serious pigment interferences. 3.2 EFFECTS OF ACCELERATED AGING ON AMINO ACID COMPOSITION OF TEMPERA PAINTTables 3 and 4 list the results from the study of the effects of accelerated aging on rabbit skin glue and egg yolk paints, respectively. It should be noted that, as a result of a peak overlap of threonine with oleic acid ethyl ester, the data for threonine in egg yolk paints are inaccurate. However, the concentration of oleic acid is greatly reduced with light or thermal aging; hence the threonine data for the aged paints are closer to the expected values. Oleic acid may be extracted from the hydrolysate prior to derivatization by treatment with chloroform (Schilling et al. 1996), but this step was not performed in order to simplify the derivatization procedure. As mentioned earlier, the data for methionine exhibit greater variability due to the absence of 2-mercaptoethanol stabilizer during hydrolysis. TABLE 3. AMINO ACID WEIGHT PERCENT COMPOSITION OF AGED RABBIT SKIN GLUE TEMPERA PAINTS TABLE 4. AMINO ACID WEIGHT PERCENT COMPOSITION OF AGED EGG YOLK TEMPERA PAINTS The results in tables 3 and 4 indicate that aging had a small effect on the total protein content of animal glue paints, whereas the protein content of egg yolk paints decreased substantially after aging (especially paints that were exposed in the Weather-o-meter). Aging caused a 25% reduction in protein content (the total weight percentage of all amino acids detected) relative to paint stored under ambient conditions. This result is due in part to the fact that egg contains a smaller weight percentage The amino acid profiles for rabbit skin glue paints matched unpigmented, unaged glue with a correlation coefficient greater than 0.994, indicating a high degree of similarity. In contrast, the correlation coefficients between the amino acid composition data of aged egg yolk tempera paints and unaged, unpigmented egg yolk were as low as 0.78, indicating that considerable changes in composition had occurred during aging. In general, egg yolk paints were more affected by heat and light than were glue paints, regardless of the pigment present. Light-aging produced the most extreme changes in amino acid concentration for both media. Table 5 lists the stable amino acid percentage data for the aged paints. Relative standard deviations for the set of data averaged 9% for all amino acids. Correlation coefficients comparing the data for the aged paints to unaged, unpigmented media were greater than 0.93 for all paint samples. The results presented in table 5 show that stable amino acid molar percentages are much less affected by the effects of aging than are data normalized to the yield of all detectable amino acids. TABLE 5. STABLE AMINO ACID MODEL PERCENT COMPOSITION OF AGED PAINT FILMS 3.3 ANALYSIS OF SAMPLES FROM OBJECTS OF ARTListed in tables 6, 7, and 8 are the data from the analysis of samples of paint and ground removed from art objects and the paints made from lead white mixed with rabbit skin glue and whole egg. The molar percentage data for all amino acids detected are listed in table 6. Stable amino acid composition data are listed in table 7. Correlation coefficient data that indicate the degree of similarity between the stable amino acid composition data of the objects with the data for selected proteinaceous reference materials are presented in table 8. The data presented in the tables for proteinaceous reference materials were selected from the listings in appendices 3 and 4. Coefficients of correlation were calculated between the object data and the data for each of the materials listed in appendix 4. For simplicity, only those materials that gave correlation coefficients greater than 0.9 were included in tables 6, 7, and 8. TABLE 6. COMPOSITION OF SAMPLES FROM OBJECTS AND REFERENCE MATERIALS, AS DETERMINED BY GAS CHROMATOGRAPHIC ANALYSIS OF AMINO ACID-ETHYL CHLOROFORMATE DERIVATIVES TABLE 7. COMPOSITION OF SAMPLES FROM OBJECTS AND REFERENCE MATERIALS, AS DETERMINED BY GAS CHROMATOGRAPHIC ANALYSIS OF AMINO ACID-ETHYL CHLOROFORMATE DERIVATIVES TABLE 8. CORRELATION COEFFIECIENTS FOR SAMPLES FROM OBJECTS AND REFERENCE MATERIALS, BASED ON STABLE AMINO ACID COMPOSITION The first example is a sample of ground from Portrait of a Man (late 15th century), a panel painting by Petrus Christus from the collection of the Los Angeles County Museum of Art (accession number 44.2.3). The painting was the subject of an in-depth technical analysis that was described in a presentation at a symposium on Petrus Christus, held in 1994 at the Metropolitan Museum, New York (Fronek 1994). The results of GC analysis showed the amino acid composition of the ground is typical for collagen (tables 6 and 7). The correlation coefficient of 0.998 (table 8) with respect to the data for collagen indicates the sample was not contaminated with egg tempera from the paint layers to any appreciable degree. It is not possible to identify the source of the collagen (such as rabbit skin glue, fish glue, or gelatin) based on the amino acid data. Extraneous peaks in the chromatogram (fig. 1), associated with fatty acids originating from lipids (Nowik 1995; Schilling et al. 1996), indicate that oil was also present in the sample. From an examination of paint cross sections, it was concluded that the oil was present in the imprimatura layer (Fronek 1994).
In a comprehensive study of the distemper paintings of Andrea Mantegna (Rothe 1992), medium identification, helpful in understanding the artist's technique, was undertaken using low-power microscopic examination, solubility tests, and various analytical techniques. Paint and ground samples removed from a large number of distemper paintings were used as study materials. The study resolved some of the common misconceptions about the media A sample consisting of brown paint removed from Mantegna's St. Mark (1448–49, St�delsches Kunstinstitut, Frankfurt) was found to contain high concentrations of alanine, hydroxyproline, and proline. The correlation coefficient data confirm the identity of the medium as collagen (table 8), although it is not clear why the glycine content is so much lower than expected. The result was in agreement with recent findings, based on solubility tests, which concluded that the medium was not egg yolk (Rothe 1992). GC analysis was performed on a sample of green paint from another Mantegna painting, Holy Family with St. Mary Magdalene (1495– 1505, catalog number 55 in the Altman Collection of the Metropolitan Museum of Art). No hydroxyproline was detected in the green (tables 6 and 7), thereby eliminating collagen as a possible medium (and also other materials that contain hydroxyproline). The correlation coefficient data (table 8) indicated the amino acid composition of the medium correlated closely to the composition of the amino acids in egg (and also the absurd materials rice and moth). The high protein content of the paint confirmed the presence of egg in the sample (it greatly exceeded that of rice). The ECF results for Holy Family with St. Mary Magdalene were inconsistent with earlier studies stating that egg is not present (Stulik 1991). This conclusion was based primarily on gas chromatography–mass spectrometry (GC-MS) results, which showed the absence of detectable quantities of cholesterol in a sample containing green and brown paint. However, the proportion of egg in the sample may have been much lower than anticipated due to the The ECF procedure was used to analyze a sample of red paint from the wall paintings of the tomb of Nefertari, located in the Valley of the Queens in Luxor, Egypt. Using various techniques, primarily carbohydrate analysis, the medium was identified in an earlier study as a type of gum arabic that was (and is still, today) available in and around Luxor (Stulik et al. 1993). This identification was supported by the amino acid composition data obtained from the ECF procedure (tables 6–8). The correlation coefficient data showed close correlation to either gum guar, gum arabic from Luxor, or lead white paint made with 5% rabbit skin glue mixed with whole egg; the hydroxyproline content of the paint is nearest to that of gum arabic from Luxor. As mentioned previously (Schilling et al. 1996), significant amounts of hydroxyproline are present in many plant gums, which may cause them to be mistakenly identified in paint samples as animal glue or gelatin. The final examples illustrating the utility of the ECF procedure are samples of ground and paint taken from painted altarpieces in Minas Gerais, Brazil (Souza et al. 1992). According to old recipes the most probable medium in the ground was parchment glue. Of particular importance is the fact that Fourier transform infrared spectrometry (FTIR) could not detect protein in any of the samples, indicating that the protein content of the grounds was below the FTIR detection limit of approximately 10% (Souza 1993). The protein in a ground sample from one of the altarpieces (CA134) correlates well to collagen (tables 6 and 7). The composition of a paint sample (CA046) comprised of particles of ground mixed with green and black paint is consistent with admixtures of collagen and egg. The correlation coefficient data indicate that the amino acid composition does not match that of any single proteinaceous medium (table 8). However, the correlation coefficient and ratio data match the results for the 2% glue/98% whole egg paint test mixture extremely well, suggesting that a mixture of the two media may indeed be present. This finding illustrates that the mixture data may aid in the identification of protein in paint samples from objects of art. |