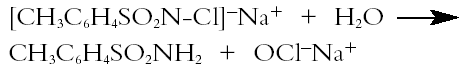SHORT COMMUNICATION: MICRO-RAMAN IDENTIFICATION OF BLOOM FORMED ON A HISTORICAL PRINT ARTIFACTVINCENT OTIENO-ALEGO, JENNIFER HODGEMAN, & DUDLEY C. CREAGH
ABSTRACT—An 1876 engraved bird's-eye view of Adelaide, the Calvert Panorama, was submitted to conservators after large quantities of loose white powder were noticed on the surface of the print and the overlying glazing. Previous conservation treatment had occurred in 1972, but no detail was given of the treatment processes. To establish what past treatments may have been applied and to prevent possible exposure to toxic materials, scientific analysis of these solids was performed. As described in this article, micro-Raman spectroscopy was employed to characterize the solid particles that composed the bloom. The unparalleled high spatial resolution (approximately 1 �m) of the technique ensured individual compound identification from a mixture of 4-60 �m-sized particles. No sample preparation was required. The analysis revealed that the bloom was predominantly paratoluenesulfonamide (the residue of chloramine-T, a chemical popular for bleaching paper in the 1960s and 1970s) and calcite, arising from the original deacidifier (calcium hydroxide). Traces of three other species—carbon black, yellow quartz, and cellulose—were also observed. None of the solids identified was a toxic pesticide residue as had been feared. TITRE—L'identification par spectroscopie de micro-Raman d'une efflorescence form�e sur une gravure historique. R�SUM�—Le Panorama de Calvert, une gravure datant de 1876 et repr�sentant une vue d'oiseau de la ville d'Adela�de, a �t� apport�e aux restaurateurs apr�s que de grandes quantit�s de poudre blanche aient �t� not�es sur la surface de l'image et sur le verre protecteur. La gravure avait d�j� �t� trait�e en 1972, mais aucun d�tail des processus de traitement n'avait �t� conserv�. Afin d'�tablir quels traitements avaient �t� appliqu�s et d'�viter que quelqu'un ne soit expos� � des mat�riaux possiblement toxiques, on a proc�d� � l'analyse �l�mentaire de ces particules par spectroscopie de micro-Raman. La r�solution spatiale �lev�e et in�gal�e (approximativement 1 �m) de cette technique a permis l'identification individuelle des composants du m�lange de particules, dont la taille varie de 4 � 60 �m. Aucune pr�paration pr�alable des �chantillons n'a �t� n�cessaire. L'analyse a indiqu� que l'efflorescence �tait compos�e essentiellement de paratoluenesulfonamide (le r�sidu de chloramine-T, un produit chimique pour blanchir le papier tr�s populaire dans les ann�es 60 et 70) et de calcite, issue du d�acidifiant initial (hydroxyde de calcium). Les traces de trois autres �l�ments—noir de charbon, quartz jaune et cellulose—ont �galement �t� identifi�es. Aucun des solides identifi�s ne s'est av�r� �tre un r�sidu toxique de pesticide comme on l'avait craint. TITULO—Identificaci�n por medio de espectroscop�a micro-Raman de un florecimiento formado sobre una estampa hist�rica. RESUMEN—Un grabado fechado en 1.876, de una vista a vuelo de p�jaro de Adelaide, el Panorama Calvert, fue remitido a unos conservadores despu�s de que grandes cantidades de polvo blanco suelto eran evidentes sobre la superficie de la estampa y del vidrio que la cubr�a. La estampa hab�a sido sometida previamente a un tratamiento de conservaci�n en 1.972, pero no se dieron detalles del proceso. Para establecer o definir que materiales se hab�an aplicado en los tratamientos previos y para prevenir una posible exposici�n a materiales t�xicos, se realiz� un an�lisis cient�fico de estos materiales s�lidos. Como se describe en este art�culo, la t�cnica de espectroscop�a micro-Raman fue empleada para caracterizar las part�culas s�lidas que compon�an el florecimiento. La alta resoluci�n espacial sin precedentes de esta t�cnica (aproximadamente 1mm), asegur� la identificaci�n individual de cada compuesto proveniente de una mezcla de part�culas de tama�os de 4 a 60 mm. No se requiri� de una preparaci�n de las muestras. El an�lisis revel� que en la composici�n del florecimiento predominaba el sulfonamida de tolueno (el residuo de la Cloramina T, compuesto qu�mico usado popularmente para blanquear papel en los a�os 60 y 70) y la calcita, proveniente del desacidificador original (hidr�xido de calcio). Tambi�n se observ� trazas de otras tres especies: carb�n negro, cuarzo amarillo y 1 INTRODUCTIONThe original black-and-white bird's-eye print forms part of a large pictorial collection of the Adelaide City Archives, Adelaide, South Australia. Originally drawn by A. C. Cooke (1836-1906) (Kerr 1992) and engraved by L. Y. S. Calvert (1828-1913) in 1876 (Kerr 1992), the bird's-eye print, or Calvert Panorama, as it is often referred to, depicts an aerial view of Adelaide city with its picturesque Mount Lofty ranges in the background (fig. 1). This particular print appeared as a supplement in the Adelaide Illustrated News of July 1876 and has been used extensively as a resource for heritage and historical inquiries. During a recent general inspection of the print, some whitish powder was observed on its surface as well as on the overlying glazing. The print also exhibited overall mottling and local areas of brownness. While the print was being deframed, a powdery material was observed to be loosely attached to the print surface, and it could easily be dislodged by gentle brushing. An annotation referring to a previous conservation treatment in 1972 was also noted. Unfortunately no details were given of the treatment. Therefore, before commencement of the new treatment, a scientific analysis of the loose solids was undertaken to assist in identifying previous treatment. In particular, this type of analysis assisted in resolving health and safety issues related to the potential toxicity of the powder (which might have contained pesticides). Analysis of bloom on art objects has used a number of techniques, including differential scanning calorimetry (DSC) (Williams 1989; Burnstock et al. 1993; Singer et al. 1995), infrared microscopy (IR) (Williams 1989; Burnstock et al. 1993), gas chromatography coupled to mass spectrometry (GC-MS) (Burnstock et al. 1993; Singer et al. 1995), scanning electron microscopy (SEM) (Burnstock et al. 1993), and energy-dispersive X-ray analysis (EDX) (Burnstock et al. 1993; Singer et al. 1995). In this study, Raman microscopy was used to analyze white powder trails observed on the bird's-eye print. This technique has been widely used in the past few years to identify pigments present in art objects (e.g., Bruni et al. 1999; Clark 1999; Derbyshire and Withnall 1999; Edwards et al. 1999; Coupry 2000; Otieno-Alego 2000). Similar to the more frequently used technique of IR, the Raman technique also involves the irradiation of a sample by a monochromatic light source (usually a laser) to produce a particular combination of the Raman bands that reflects the vibrational modes typical of the material under examination. However, because of differing quantum mechanical selection rules, IR and Raman spectra are not identical but are said to be complementary (Schrader 1995).That is, vibrations that are strong in a Raman spectrum are usually weak in an IR spectrum and vice versa. Qualitatively, antisymmetric vibrational modes and vibrations due to polar bonds (such as O-H, N-H, C=O) generally exhibit prominent IR bands, while Raman tends to emphasize vibrations involving more symmetrical bonds (such as C=C, C-C, S-S) (Schrader 1995).
To the analyst, Raman microspectroscopy offers many benefits in comparison to IR microspectroscopy. The most important are the ease of sample preparation, sampling through glass and plastic, ability to analyze aqueous samples, and the compatibility with fiber optics allowing for remote sampling. Furthermore, the laser beam used in Raman technique can be focused to achieve a high spatial resolution (typically 1 �m), which is 20 times better than the focus achievable with IR radiation (Best et al. 1992). This high spatial resolution facilitates the
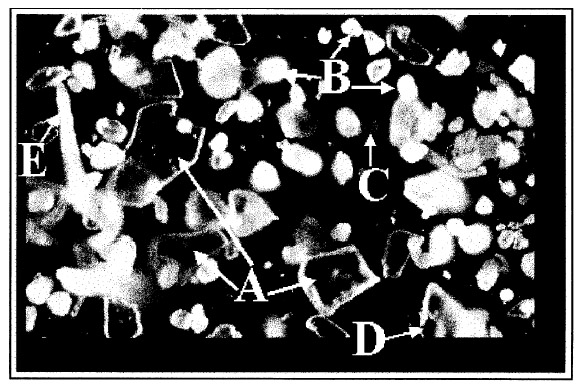
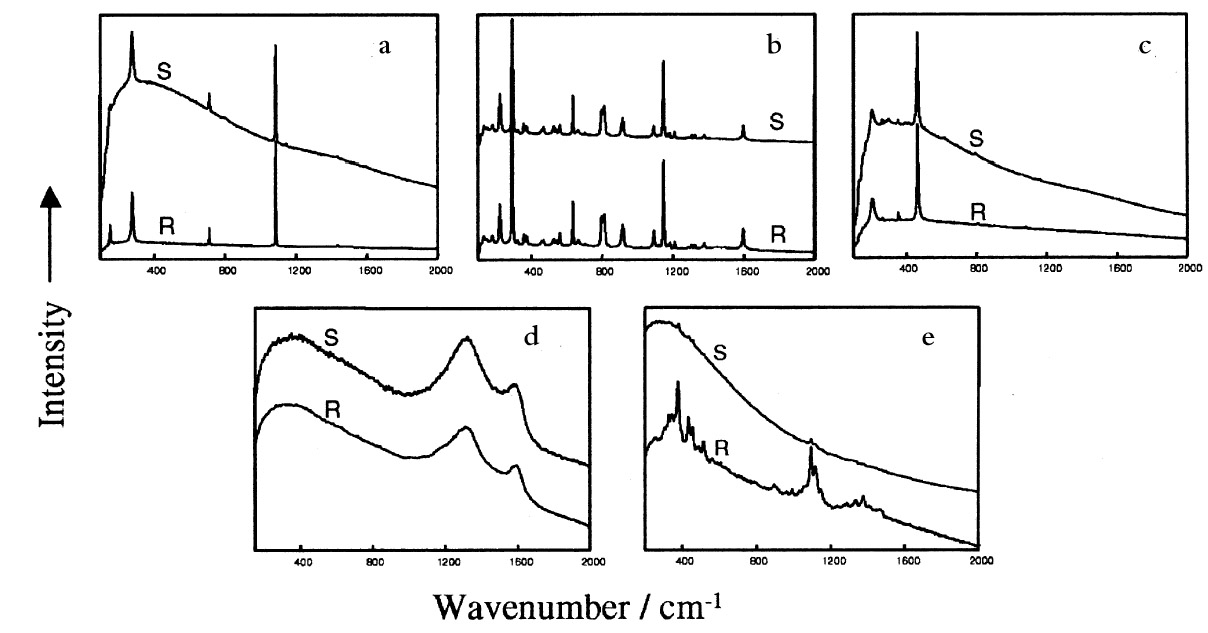
2 EXPERIMENTAL2.1 ABOUT THE ARTIFACTThe artifact, which is approximately 65 x 103 cm2 in size, has been hanging in a general manager's office for about seven years. It is framed under glass with an ornamental wooden frame, on a linen backing adhered to a laminated board. The picture itself is centered in the upper portion of the print, giving room below for the decorative printed text ADELAIDE (see fig. 1). A note attached to the frame indicates that previous conservation treatment was performed in 1972. 2.2 EXPERIMENTAL DETAILSThe powdery deposits on the print were brushed off into a sealable polyethylene bag. Preliminary examination using an ordinary light microscope showed the solid deposit to be a mixture of a number of microsized crystals. A sample of this powder (approximately 1 mg) was placed on a microscope slide for direct Raman microanalysis. Raman spectra were recorded using a Raman imaging microscope (Renishaw PLC, model 2000) coupled with an Olympus BH2 microscope. The Ramascope is equipped with an air-cooled charge-coupled device (CCD) detector, a motorized XYZ stage, and an image-viewing video camera. A near infrared laser source emitting at 780 �m was used as the excitation source. The Ramascope was set up in its confocal mode (Williams et al. 1994) for maximum spatial resolution (approximately 0.7 �m). With this setting, individual solids within the mixture were separately identified. A representative sample area of 1200 x 1200 �m2 was defined with the aid of the motorized stage. The individual particles enclosed in this area were each pinpointed using the video viewer, and the targeted solid was analyzed by focusing the laser beam via the 50x microscope objective. Integration times ranged between 20 and 40 seconds, with 5 to 10 spectral accumulations. Attempts to physically separate the individual particles for complementary analysis using infrared microscopy were unsuccessful because of the inability to focus the IR beam into a sufficiently small area. 3 RESULTS AND DISCUSSIONFigure 2 shows a typical white light micrograph of the powdery deposit examined under a light microscope using a 10x microscope objective. This examination revealed three distinct types of crystalline material. The deposit included relatively large transparent crystals (see example labeled A in fig. 2) dispersed amid the bulk of much smaller, more rounded white crystals (example labeled B, fig. 2) and a few orange-colored crystals (example labeled C). Some black particles (labeled D) were also observed, as well as white fiberlike materials (labeled E) whose lengths ranged between 50 and 200 �m. These particles hereafter will be referred to simply as solids A, B, C, D, and E, respectively. Raman microanalysis of all the solid particles enclosed in the predefined area of 1200 x 1200 �m2 was undertaken by focusing the laser onto the individual particles via the microscope objective. Identification
The occurrence of paratoluenesulfonamide may be linked to the use of chloramine-T (the sodium derivative of N-chloro-p-toluenesulfonamide: ([CH3C6H4SO2N(Cl)Na]), which was a popular paper bleaching reagent in the 1960s and 1970s (Lienardy and Van Damme 1990).When dissolved in ethanolic solution, chloramine-T slowly hydrolyzes, releasing hypochlorite ions (Corrigan 1997) according to the following equation:
Thus, the bleaching action of chloramine-T is comparable to that of sodium hypochlorite, except that in this case the bleaching process is much slower and therefore more controllable. The Cl-free toluenesulfonamide identified in this study is a product of this cleavage reaction. Chloramine-T bleaching was considered to be simple, efficient, safe, and less harmful to the paper (Hey 1977). Paper treated with chloramine-T required only a simple rinse because traces of the chemical were presumed to have a bactericidal action long after the treatment (Lienardy and Van Damme 1990). The occurrence of its residues on this print artifact is thus not surprising. The idea that chloramine-T is safe has since been amended. Chloramine compounds are unstable, and the residual The black carbon particles most likely came from the print ink, while the cellulose fibers broke loose from the aging paper. The source of the few quartz crystals identified in the white solid deposit is probably trapped dust/sand particles from the environment. 4 CONCLUSIONSThe high spatial resolution of the micro-Raman technique has been exploited in the individual identification of solid compounds amid a mixture of 4-60 �m-sized particles that constituted a white bloom on an 1876 print artifact. No sample preparation was required. In this instance, the powdery deposit brushed off the surface of the print artifact was simply placed on a microscope slide and directly analyzed. The Raman spectra of the individual particles were recorded and compared with those available in our spectral database for identification. The white deposit was predominantly a mixture of calcium carbonate and paratoluenesulfonamide (almost certainly arising from a previous conservation treatment by chloramine-T), with small amounts of black carbon, quartz, and microsized cellulose fibers from the paper matrix. None of the solids identified was a toxic pesticide residue, as had been feared, and the conservation treatment of this valuable artifact is now under way. It is noteworthy that the use of chloramine-T as a paper-bleaching chemical has now been discontinued due to a proven problem of severe degradation of the cellulose fiber. ACKNOWLEDGEMENTSThe authors are indebted to the Adelaide City Archives, Adelaide, South Australia, for providing permission to publish this data. REFERENCESBest, S. P., R. J. H.Clark, and R.Withnall. 1992. Non-destructive pigment analysis of artifacts by Raman microscopy. Endeavour16(2):66–73. Bruni, S., F.Cariati, F. Casadio, and I.Toniolo. 1999. Identification of pigments on a XV century illuminated parchment by Raman and FTIR microspectroscopies. Spectrochimica Acta Part A Molecular Spectroscopy55(8):1371–77. Burnstock, A., M.Caldwell, and A.Odlyha. 1993. A technical examination of surface deterioration of Stanley Spencer's paintings at Sandham Memorial Chapel. ICOM Committee for Conservation Preprints, 10th Triennial Meeting, Washington, D.C., August 22-27. Paris: ICOM. 231–38. Clark, R.J.H. 1999. Raman microscopy: Sensitive probe of pigments on manuscripts, paintings and other artefacts. Journal of Molecular Structure481(Special Issue SI):15–20. Corrigan, C.1997. Bleaching. In Old Master prints and drawings: A guide to preservation and conservation, ed.M. B.Cohn. Amsterdam: Universal Press. 264–77.
Coupry, C. L.2000. Application of Raman microspectrometry to art objects. Analysis28(1):39–46. Derbyshire, A., and R.Withnall. 1999. Pigment analysis of portrait miniatures using Raman microscopy. Journal of Raman Spectroscopy30(3):185–88. Edwards, H. G. M., D. W.Farwell, F. R.Perez, and S. J.Villar. 1999. Raman microscopy of a mediaeval Spanish cantoral. Applied Spectroscopy53(11):1436–39. Hey, M.1977. Paper bleaching: Its simple chemistry and working procedures. Paper Conservator2:10–21. Kerr, J., ed. 1992. The dictionary of Australian artists: Printers, sketchers, photographers and engravers to 1870. Melbourne: Oxford University Press. 127, 172. Lienardy, A., and P VanDamme. 1990. A bibliographical survey of the bleaching of paper. Restaurator9:178–98. Otieno-Alego, V.2000. Raman microscopy: A useful tool for the archaeometric analysis of pigments. In Radiation in art and archeometry, ed.D. C.Creagh and D.A.Bradley. Amsterdam: Elservier Science. 76–100. Schrader, B., ed. 1995. Infrared and Raman spectroscopy. NewYork: VCH. Singer, B., J.Devenport, and D.Wise. 1995. Examination of a blooming problem in a collection of unvarnished oil paintings. Conservator19:3–9. Williams, K. P. J., G. D.Pitt, D. N.Batchelder, and B. J.Kip.1994. Confocal Raman microspectroscopy using a stigmatic spectrograph and CCD detector. Applied Spectroscopy48(2)::232–35. Williams, S. R.1989. Blooms, blushes, transferred images and mouldy surfaces: What are these distracting accretions on art works? Proceedings of the 14th Annual IIC-Canadian Group Conference, Toronto, Canada. Ottawa, Ont.: IIC-Canadian Group. 65–84. SOURCES OF MATERIALSRenishaw Raman Imaging MicroscopeRenishaw PLC, Spectroscopy Products Division Old Town, Wotton-Under-Edge Gloucestershire GL 12 7DW UK AUTHOR INFORMATIONVINCENT OTIENO-ALEGO earned his B.Ed. (Science) and M.S. at Kenyatta University and his Ph.D. in electrochemistry at Queensland University of Technology. His research interests have developed in two principal directions, corrosion science and vibrational spectroscopy. Most recently, his corrosion research has been centered on the use of environmentally friendly corrosion inhibitors in conservation. In vibrational spectroscopy, he is currently using the techniques of Raman and infrared microspectroscopies to study the degradation of plastics and polymer composites, as well as to analyze artists' materials on a number of artifacts. He is a research officer at University of Canberra. Address: Raman Microscopy Unit, Division of Science and Design, University of Canberra, ACT 2616, Australia. JENNIFER HODGEMAN graduated in 1997 from the University of Canberra Conservation of Cultural Materials Program, Canberra, Australia. On graduating she worked at the Adelaide City Archives as a paper conservator. Since November 1999 she has been working at the National Library of Australia in the digital preservation section. Prior to conservation studies, she worked as a senior technical officer in the field of forensic analytical chemistry. Address: National Library of Australia, Preservation, Parkes, ACT, Australia. DUDLEY C. CREAGH has extensive experience in the development of new equipment and techniques for investigating photon scattering in the X-ray, optical, and infrared regions of the electromagnetic spectrum. He has been active in the development and use of advanced analytical techniques for the study of problems associated with the conservation of cultural heritage materials in museum custody for more than 20 years. He is expert, inter alia, in the determination
 Section Index Section Index |