EFFECTS OF DILUTE CALCIUM WASHING TREATMENTS ON PAPERJOHN BOGAARD, & PAUL M. WHITMORE
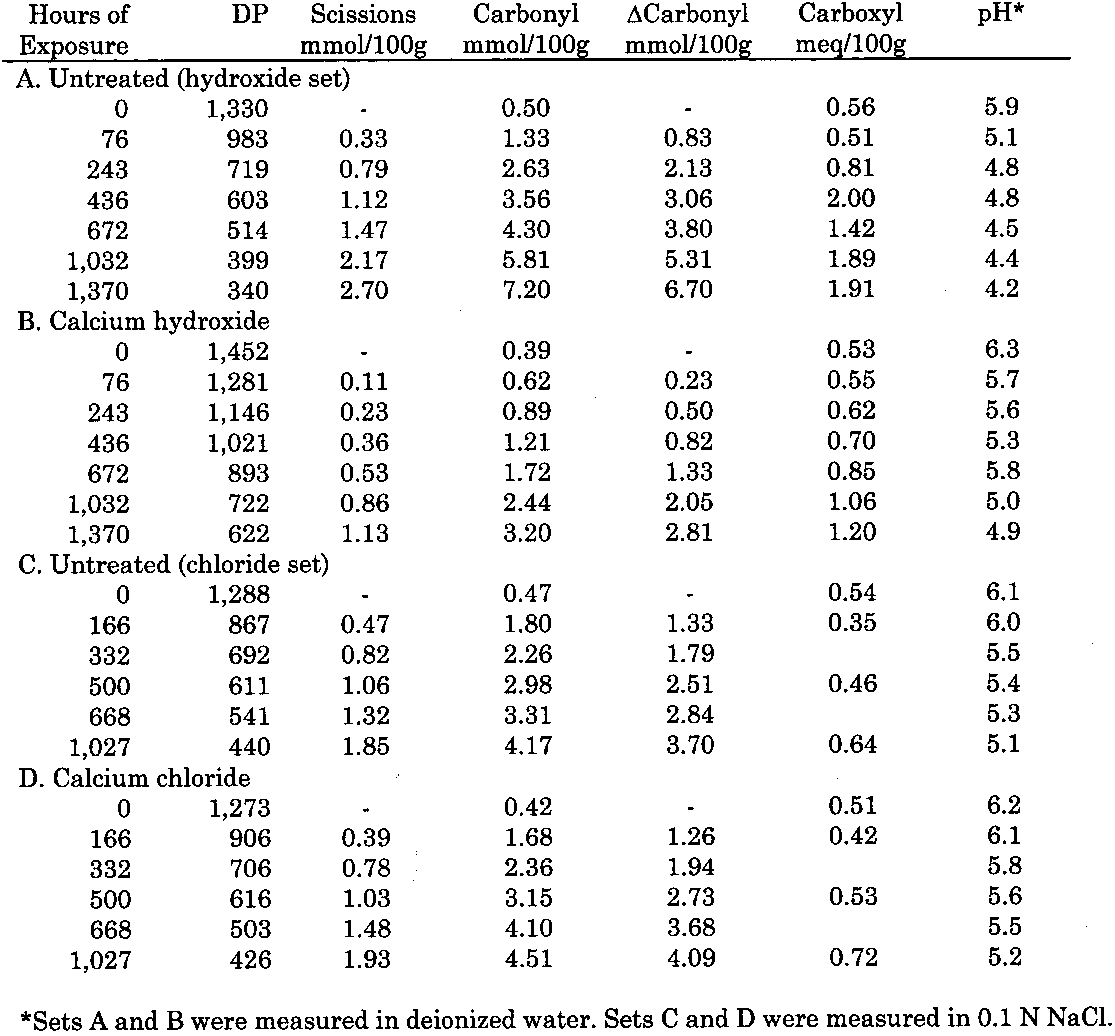
3 RESULTS3.1 UNOXIDIZED SAMPLESThe data for the treatment and thermal aging of unoxidized sheets treated with solutions of the three calcium salts are summarized in table 1. The immediate result of each treatment can be seen in the samples of each set with zero hours of aging. All the treatments caused a very slight reduction of DP (less than 4% in the worst case) as well as in the carbonyl and carboxyl contents, while the pH increased a small amount from the treatment. The results of accelerated
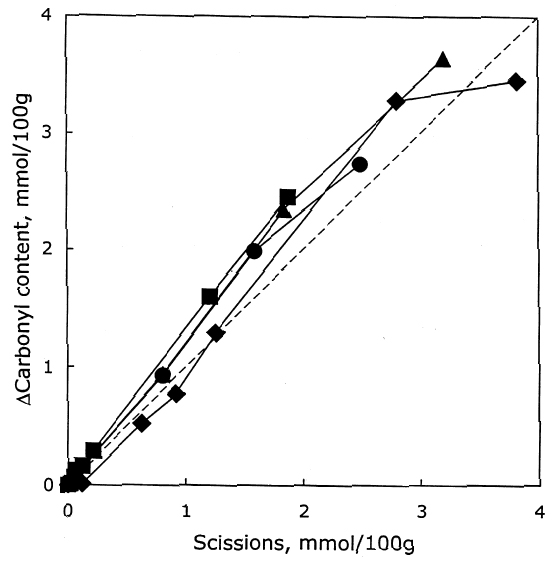
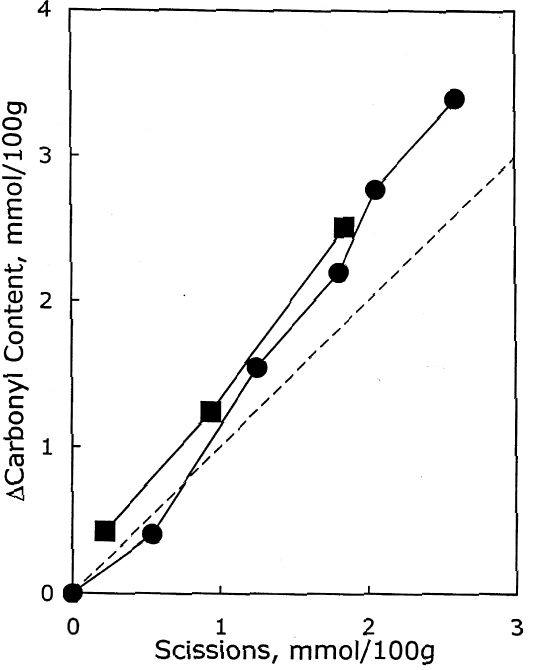
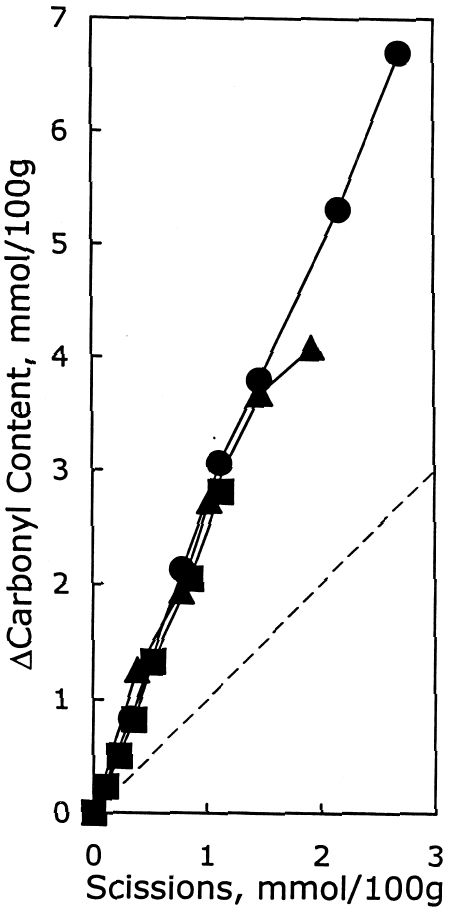
As described earlier, the chemical products of aging can be analyzed to determine the dominant degradation process. In figure 2 the scissions are graphed versus the change in carbonyl content upon aging. In accelerated thermal aging, it is normal for acid hydrolysis to be the primary degradation path-way (Whitmore and Bogaard 1994), and this behavior is demonstrated here by the approximately equal production of scissions and carbonyls in the aging of both untreated and treated sheets. So, while the treatments slowed down the degradation, and in the case of the hydroxide treatment slowed it down greatly, the dominant process is still hydrolysis. There is no significant increase in oxidation of the cellulose, which would be indicated by a greater production of carbonyls relative to chain scissions. For all these treatments, the calcium loading in the sheet is approximately equal, ranging only from 190 ppm (by weight of calcium) in the hydroxide treatment to 230 ppm in the chloride treatment. Judging by the results of the set treated with calcium chloride, the calcium itself has only a very modest beneficial effect. The stronger effect seems to be due to the greater neutralization of the sheet (measured by the increase of the extract pH) from the more alkaline bicarbonate and hydroxide solutions. Papers treated with calcium hydroxide had a period of chemical stability three or four times longer than those given the mild bicarbonate treatment. 3.2 PHOTO-OXIDIZED SAMPLES3.2.1 Characteristics of Alkaline DegradationFor the samples immersed in sodium hydroxide, the results are summarized in table 2. In figure 3 the degree of polymerization of the samples is graphed
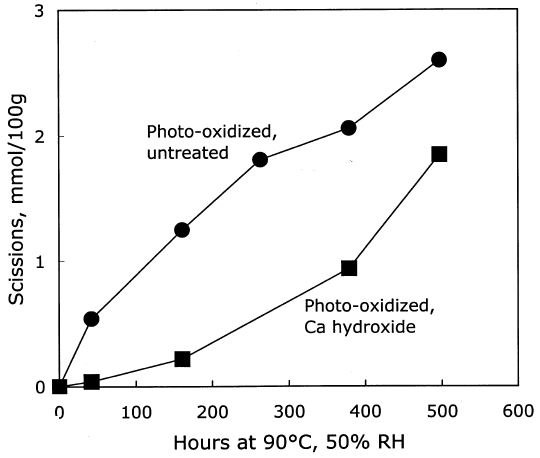
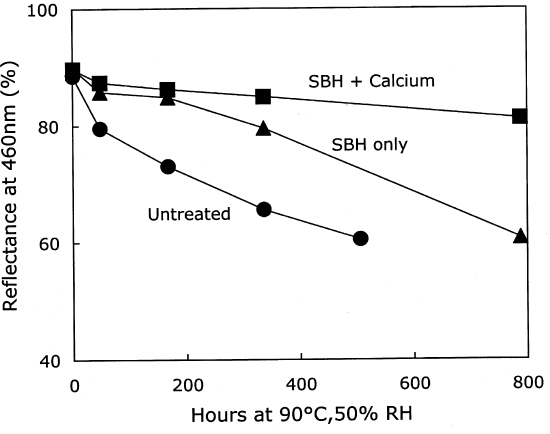
3.2.2 Treatment with Calcium HydroxideThe results of the treatment of photo-oxidized paper sheets with calcium hydroxide, and the subsequent thermal aging, are contained in table 3. Although these sheets were strongly photo-oxidized, the short immersion in the mildly alkaline bath caused only a modest amount of damage. There was a small reduction in DP of about 7%, and the carboxyl groups increased slightly. The carbonyls were virtually the same after the treatment, unlike the immersion in sodium hydroxide, which decreased their concentration. The thermal aging results are graphed in figure 5. As with the unoxidized paper, the calcium hydroxide treatment again provided an initial period of slow degradation, but the stabilizing effect was not nearly as long lasting as for the treated, unoxidized sheets in figure 1. The degradation rate of the treated sheet was much slower than the rate for the untreated, photo-oxidized sheet for about 200 hours of accelerated aging, after which the rates for both were about the same. This finding is in contrast to the effect of the calcium hydroxide treatment of the unoxidized sheets, where there was very little degradation at all for more than 1,000 hours of oven aging. In figure 6 the new carbonyl groups produced upon thermal aging are graphed versus the scissions, and both treated and untreated samples demonstrate degradation that is predominantly hydrolytic. The alkaline treatment did not seem to cause more rapid oxidation of these papers upon aging.
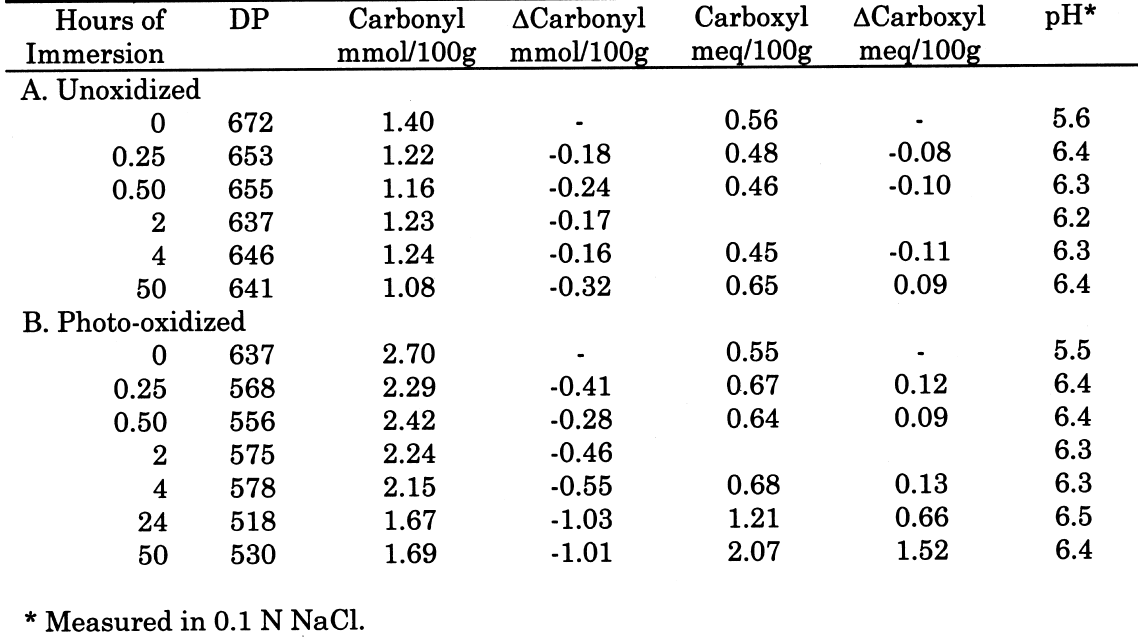
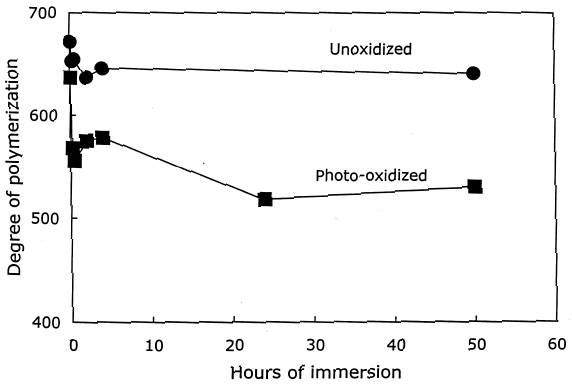
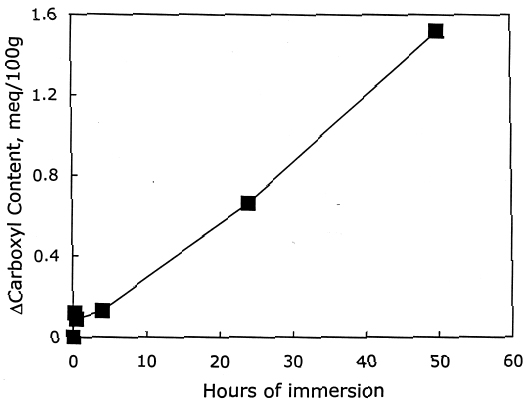
From these investigations, it seems that most of the alkaline damage to photo-oxidized cellulose occurs during immersion in the treatment bath. Yet, because of the increased oxidation, the sheet also needs a stronger alkaline treatment to significantly slow the subsequent acid hydrolysis. 3.2.3 Pretreatment with Sodium BorohydrideThe sodium borohydride treatment is designed to protect alkali-sensitive linkages by converting the vulnerable carbonyl groups to less reactive hydroxyls. Yet, the borohydride solution is alkaline itself (pH = 9.5), so until the reduction is complete, some damage may occur. The results of the treatment of photo-oxidized papers, summarized in table 4, show a very minor amount of alkaline degradation from the borohydride immersion, as evidenced by the lowering of DP by less than 5%. Along with this minor degradation is the expected large reduction in carbonyl content and a very small increase in carboxyl groups, as well as a significant degree of neutralization. The subsequent calcium hydroxide treatment did not cause any significant further lowering of DP and neutralized the sample sheets a little more. None of the treated samples were left with an alkaline extract pH.
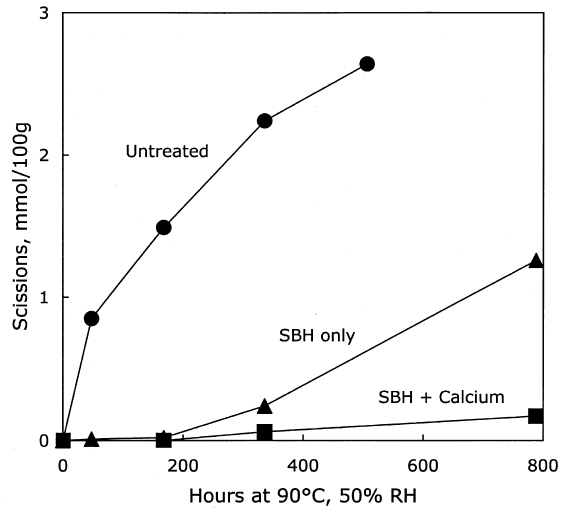
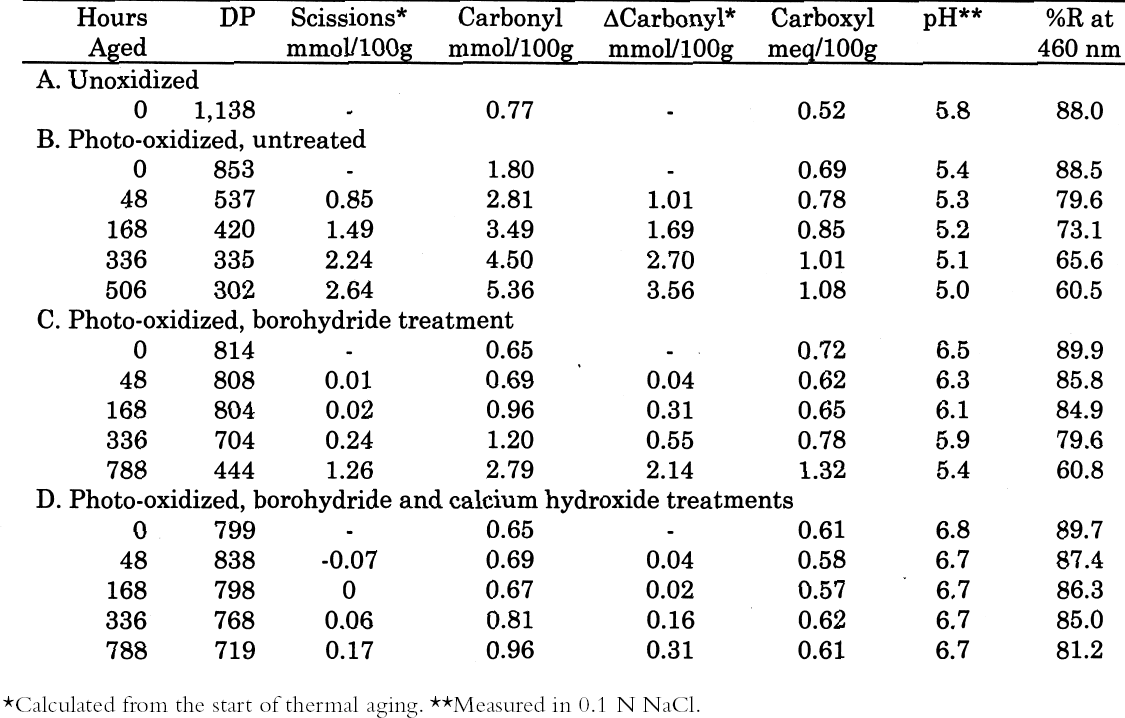
The thermal aging results are graphed in figure 7. The photo-oxidized but untreated sample set deteriorated very quickly, while the chemically reduced papers had a relatively long period of chemical stability. Eventually, the stabilization imparted by the reduction treatment alone seemed to run out, and the scission rate increased as the pH fell. However, the samples that had received the reduction treatment followed by calcium hydroxide washing demonstrated excellent chemical stability during the entire period of accelerated aging. The stability was far greater than the calcium hydroxide treatment alone on the photo-oxidized sheets, shown in table 3 and figure 5. In fact, the result of the combined borohydride-hydroxide treatment of photo-oxidized paper was comparable to the stabilizing effect of the calcium hydroxide treatment of unoxidized paper shown in figure 1. In addition to the slower reduction in DP, the
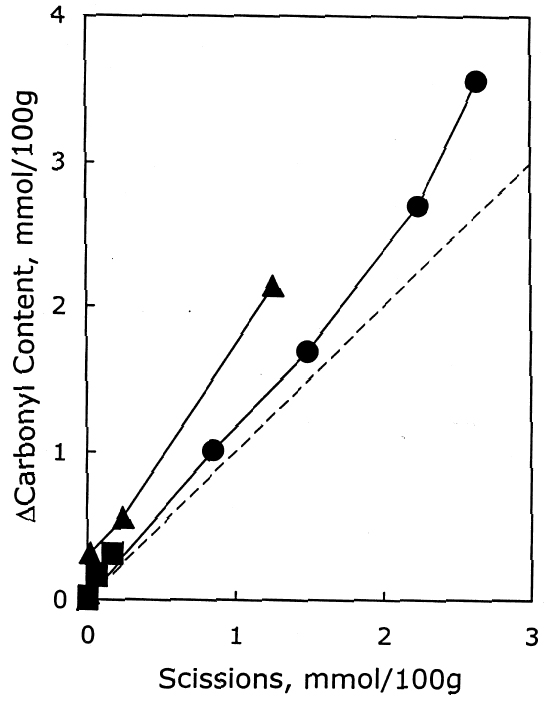
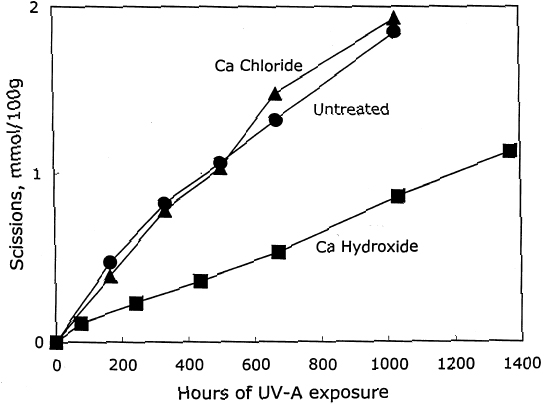
After again examining the ratio of carbonyls and scissions produced during aging (shown in figure 9), acid hydrolysis seems to be the primary degradation pathway here. However, for the reduction-only samples, there is a small amount of extra oxidation produced upon thermal aging, which is also indicated by the increase in carboxyl content. The borohydride-hydroxide—treated samples degraded so little during the oven aging that it is difficult to determine whether they, too, exhibit a small amount of oxidative chemistry. 3.3 EFFECT ON PHOTO-OXIDATIONThe results for unaged filter papers treated with calcium hydroxide or chloride, then exposed to ultraviolet-A light, are shown in table 5. The samples treated with calcium hydroxide were somewhat neutralized, whereas the chloride treatment caused only slight neutralization. The treatments otherwise resulted in only very minor changes in DP and carbonyl and carboxyl content. As illustrated in figure 10, the rate of scissions produced for the calcium hydroxide—treated samples is about one-third that of both the untreated set and the calcium chloride—treated set, which are virtually identical. The other notable feature of this graph is that the degradation rates are steady over the whole period of exposure, unlike the thermal aging, in which the alkali-treated sample had a period of stability followed by a gradual increasing of rate. For the photo-oxidizing sheet, there is no evidence of a protective component being exhausted.
The degradation products are graphed in figure 11, which shows a ratio of about 2.7 new carbonyl groups produced for every scission, which has been observed in a prior study of photo-oxidation of untreated sheets (Whitmore and Bogaard 1994). This product ratio is the same for both the untreated and the treated samples, indicating that the treatments are
|