THE EARLY PAINTED ENAMELS OF LIMOGES IN THE WALTERS ART MUSEUM: HISTORICAL CONTEXT AND OBSERVATIONS ON PAST TREATMENTSTERRY DRAYMAN-WEISSER
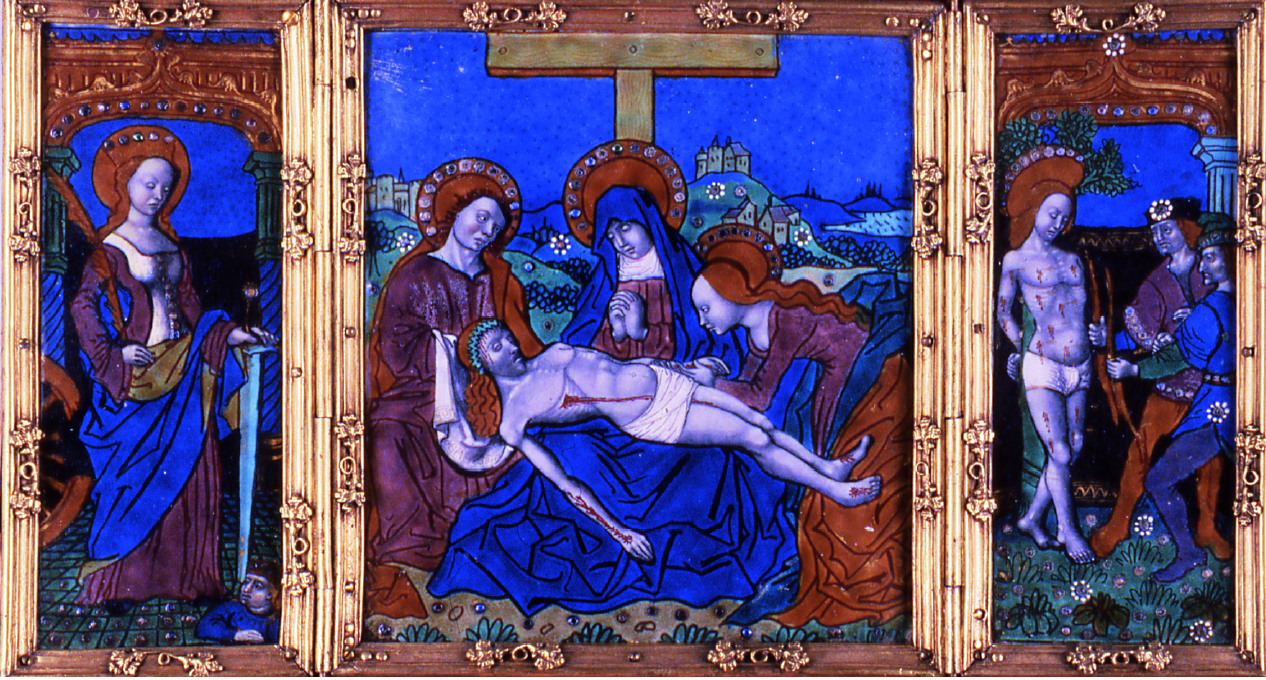
ABSTRACT—The Walters Art Museum has one of the most significant collections of enamels in the world and one of the largest collections of painted enamels from Limoges. The early painted enamels, dating from the late 15th and early 16th centuries, suffer from inherent chemical instability leading to weeping, crizzling, and ultimately the breakdown of the enamel surfaces. Currently there are no generally accepted treatments for arresting this deterioration. Conservators at the Walters have carried out treatments in the past, attempting to stabilize the actively deteriorating enamels. This article places the early painted enamels from Limoges in their historical context, describes the techniques of their manufacture, reviews the literature on the causes of their deterioration, and describes and evaluates the treatments carried out at the Walters to stabilize them. TITRE—Les �maux peints de Limoges de type ancien au Walters Art Museum: contexte historique et observations sur les traitements ant�rieurs. R�SUM�—Le Walters Art Museum (Mus�e d'art Walters) poss�de l'une des plus importantes collections d'�maux dans le monde et l'une des plus grandes collections d'�maux peints de Limoges. De fa�on inh�rente, les �maux peints datant de la fin du 15�me et du d�but du 16�me si�cle souffrent d'instabilit� chimique, ce qui cause des suintements, des craquelures et finalement la d�sint�gration des surfaces en �mail. Actuellement il n'y a aucun traitement g�n�ralement adopt� pour arr�ter cette d�t�rioration. Les restaurateurs du mus�e ont effectu� par le pass� plusieurs traitements en vue de stabiliser les �maux qui �taient visiblement en train de se d�t�riorer. Cet article discute du contexte historique des premiers �maux peints produits � Limoges et d�crit leurs techniques de fabrication. Il offre aussi une revue de la litt�rature sur les causes de leur d�t�rioration, et enfin d�crit ainsi qu'�value les traitements de stabilisation effectu�s au Walters Art Museum. TITULO—Los esmaltes tempranos de Limoges en The Walters Art Museum (Museo de arte Walters): Contexto hist�rico y observaciones sobre tratamientos pasados. RESUMEN—El Walters Art Museum (Museo de arte Walters) tiene una de las colecciones de esmaltes m�s notables del mundo, y una de las m�s grandes colecciones de esmaltes pintados de Limoges. Los esmaltes pintados m�s antiguos, que datan de fines del siglo XV y principios del siglo XVI, presentan inestabilidad qu�mica inherente, que conduce a la formaci�n de gotas de humedad en la superficie (weeping), la formaci�n de una red fina de grietas (crizzling) y por �ltimo, a la desintegraci�n de la superficie de los esmaltes. Actualmente no hay tratamientos aceptados de manera generalizada para detener este tipo de deterioro. En el pasado, los conservadores del Museo Walters llevaron a cabo tratamientos que intentaban estabilizar el deterioro activo de los esmaltes. Este art�culo ubica a los esmaltes tempranos de Limoges en su contexto hist�rico, describe las t�cnicas de manufactura, hace una rese�a de la literatura existente sobre las causas de su deterioro, y describe y eval�a los tratamientos llevados a cabo en el Museo Walters para estabilizarlos. TITULO—Esmaltes antigos pintados de Limoges no Walters Art Museum (Museu de Arte Walters): contexto hist�rico e observa��es de tratamentos anteriores. RESUMO—Walters Art Museum (Museu de Arte Walters) possui uma das mais significativas cole��es de esmaltes do mundo e umas das maiores cole��es de esmaltes pintados de Limoges. Datando do final do s�culo XV e princ�pio do s�culo XVI, os esmaltes antigos pintados sofrem instabilidade qu�mica inerente que ocasiona a forma��o de bolhas de �gua, opacidade gerada por mudan�a da textura da superf�cie e finalmente o craquelamento da superf�cie do esmalte. Atualmente n�o existe nenhum tratamento aceit�vel que detenha esta deteriora��o. No passado, restauradores do Walters realizaram tratamentos visando estabilizar os esmaltes em processo de deteriora��o. Este artigo situa os esmaltes antigos pintados de Limoges no seu contexto hist�rico, descreve as t�cnicas de sua manufatura, revisa os textos t�cnicos sobre as causas 1 INTRODUCTIONIn his treatise Pirotechnia, the 16th-century master craftsman and metalworker Vannoccio Biringuccio said of enamel and glass: “Considering its brief and short life … it cannot and must not be given too much love, and it must be used and kept in mind as an example of the life of man and of the things of this world which, though beautiful, are transitory and frail” (quoted in Smith and Gnudi 1990, 132). Many Renaissance enamels survive today in almost pristine condition. Biringuccio undoubtedly would be surprised but pleased to find that these enamels created in his lifetime are still being admired for their beauty 500 years later. However, some Renaissance enamels indeed tend to be “transitory and frail.” A group of these less durable enamels, the early painted enamels of Limoges, are now in the collections of the Walters Art Museum (fig. 1, see page 252). The technology, deterioration, and condition of these enamels are the focus of this study. Since attempts have been made to stabilize these actively deteriorating objects in the past, an evaluation of the success of these treatments will be presented. Produced in Limoges in central France beginning around 1470, the early painted enamels are vividly colored pictorial images of religious subjects on rectangular copper plaques usually less than 27 cm in height. Often three plaques were created at once and grouped together in a frame to form a triptych. The original frames generally have been lost, and most of the enamels were remounted in the 19th and early 20th centuries in wood or fabric-covered wood and metal frames, probably to increase their salability for the art market. From the time the early painted Limoges enamels entered the Walters collection, they exhibited active deterioration phenomena such as crizzling, weeping, flaking, and salt efflorescence (figs. 2–4; see page 252 for fig. 3). The result has been loss of translucency, cracking, and pitting of the enamel surfaces. Similar deterioration has been noted in early painted Limoges enamels in other collections. 1 Thus the problems appear to be inherent in the enamels themselves, rather than related to poor handling or improper treatment. Philippe Verdier, who cataloged the Walters, Frick, and Taft Museum collections of painted enamels, commented, “Most of the enamels of the 1500s are partly eroded to the point of looking powdery or dusty” (Verdier 1957, 45).
The end of the early painted enamel period is generally accepted to be 1530, as several changes took place around that time. Strictly religious themes gave way to a mixture of religious and mythological themes, and the enamels from Limoges, no longer strictly devotional, became increasingly popular as luxury items. Vessels, plates, and other forms, in addition
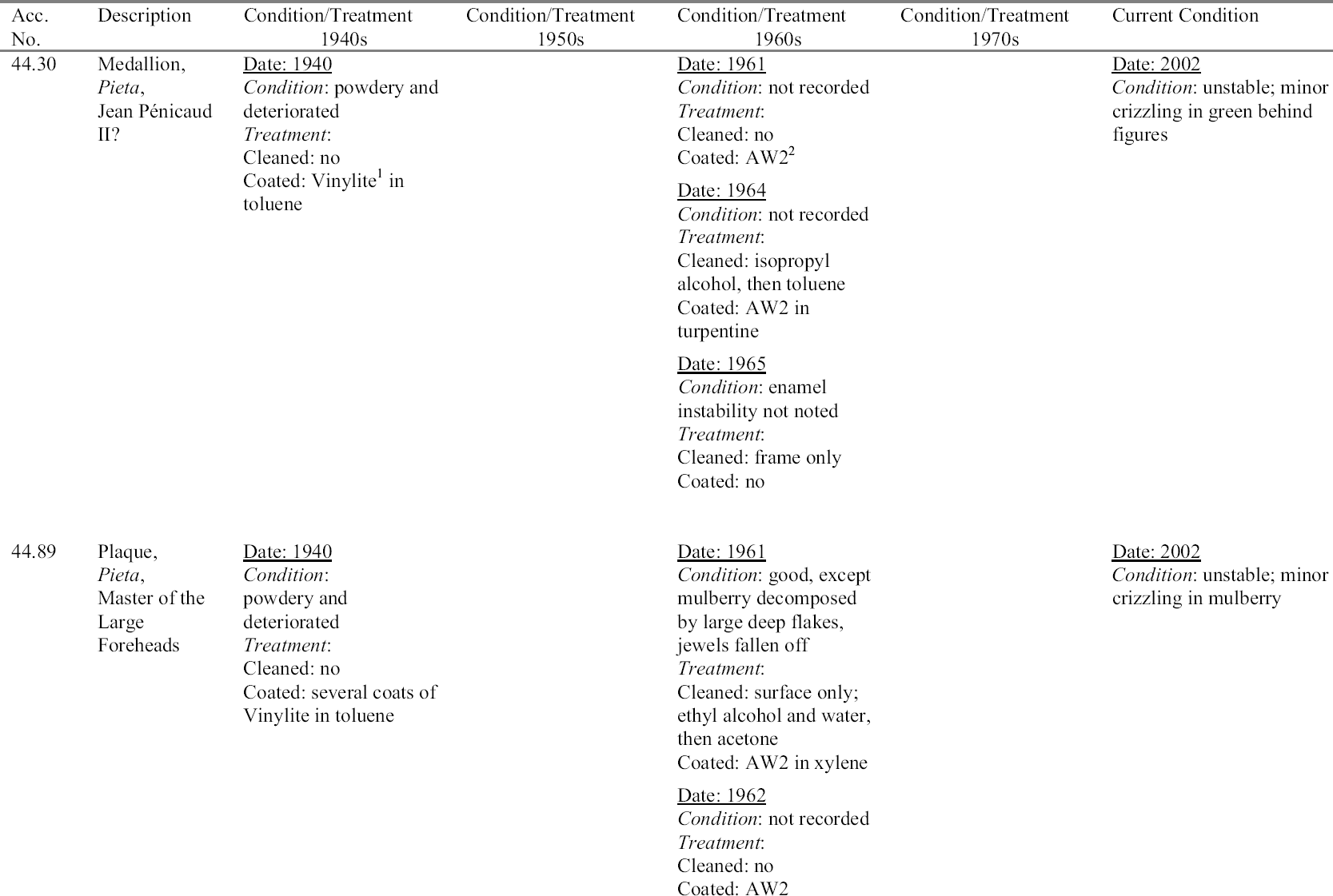
In the early 1960s Peter Michaels, a conservator at the Walters, developed and carried out a treatment on most of the unstable early painted Limoges enamels in the collection. The treatment generally involved washing the enamels with water followed by coating with a synthetic resin. This treatment restored the translucency of the enamels and appeared to stabilize them. Since that time, our understanding of the deterioration mechanisms in unstable glass and enamel has grown, and current conservation philosophy favors noninvasive treatment whenever possible. Therefore, from today's perspective, Michaels's treatments may seem intrusive, unusually risky, and perhaps more likely to accelerate than inhibit deterioration of the enamel. In an article on the deterioration of painted Limoges enamel plaques, Smith et al. questioned whether past treatments, including those by Michaels, will be effective over time (1987). After more than 40 years, it seems an appropriate time to evaluate the results of Michaels's treatments.
2 PRECURSORS TO THE EARLY LIMOGES PAINTED ENAMELSHerbert Maryon, in his treatise Metalwork and Enamelling (1971, 169), defines enamel “as a vitreous coating fused to a metallic base.” The process of One of the earliest literary references to enameling appears around A.D. 200 in the writings of Philostratus of Lemnos. While describing a boar hunt, he mentions the decoration on horse trappings in this way: “They say that the Barbarians who dwell in Ocean pour these colors on heated bronze, and that they adhere, become as hard as stone and preserve the designs that are made on them” (quoted in Chamot 1930, 1). It is interesting to note that national rivalries concerning enamels may influence interpretations of writings on the subject. For example, regarding Philostratus's observations, Chamot (1930, 1) writes in her book on English medieval enamels: “Some French writers have tried to prove that the country referred to was Gaul, but it is on the whole more likely that Britain was intended, especially since the finest specimens of early champlev� enamel on bronze have been found in this country.” From ancient times to the Renaissance, enamel was applied to metal in a number of ways, often achieving similar effects. One element that is common to all the techniques is that some metal remains visible on the surface, usually playing a decorative role in the design. For the craftsmen producing these works, goldsmithing and metalworking skills were as important as expertise in enameling. According to medievalist Marvin Ross (1941, 32), “All during the Middle Ages the working of metal and enameling were closely allied. In fact, Limoges … had no separate guild for its enamelers and they were included with the metalworkers. This seems quite just, because from the very nature of enameling no craftsman could practice without first being a metalworker.” The fact that goldsmithing and enameling were associated crafts is supported by documents dated 1328, showing that the goldsmith, Etienne de Salinas, re-enameled a jar and gold goblet in Paris for Mahaut, countess of Artois (Barsali 1969). Gold, silver, and copper and its alloys have been used most often as the metal support for enameled works of art. The major elements of the vitreous or glass layer are generally silica (from sand or quartz pebbles), an alkali-rich flux (derived from plant or wood ash, natron, and saltpeter), alkaline earth stabilizers (often naturally associated with the flux or the silica), and metal oxides to achieve various colors (Freestone 1992). Differences in enameling techniques can affect the appearance and durability of the work of art. For this reason, and because it is important to understand what makes the painted enamel technique unique, general descriptions of enameling techniques in use preceding the production of the painted Limoges enamels are reviewed here. 2.1 FILIGREE ENAMELINGSince the time of the ancient Greeks and Etruscans, the filigree enamel method has been used to add small touches of color to decorative goldwork (fig. 6, see page 253). In this technique, decorative wires, usually twisted, are used to create surface designs, and enamel is applied to areas enclosed by the wires. The enamel is shallow and may be concave or convex since it is not ground level or polished. Since the enamel often does not adhere well to the substrate and is easily damaged, it is fortunate that even if the enamel is lost, the wires themselves are decorative and can stand on their own as design elements. This is one important feature that visually differentiates the filigree enameling technique from the cloisonn� method. 2.2 CLOISONN� ENAMELINGCloisonn� enameling (fig. 7, see page 253) was used extensively in the Byzantine period, although there are certainly earlier examples as well. It is possible that the inspiration for the development of the technique came from a desire to imitate works that were decorated with glass or stone inlays. In the cloisonn� technique, metal strips are attached on edge to the surface of a metal plate to create cells. These cells are then completely filled with enamel to create the design. The enamel is ground even with the top edges of the cell walls and polished 2.3 PLIQUE-A-JOUR ENAMELINGPlique-a-jour enameling (fig. 8, see page 253), is a variation on the cloisonn� technique. While the enamel is contained within cells, the difference lies in the fact that there is no metal plate beneath the enamel. The cells may be made with flat or decorative metal strips or by piercing a metal sheet to create open spaces. During the enameling process the cells are filled, using a temporary backing plate to keep the enamel in place during firing. The enamel is usually level with the top edges of the metal cell walls and polished. When the temporary backing plate is removed, light passes through the translucent enamel, creating a stained-glass-window effect. Due to the fragile nature of the structural support for the enamel, few early examples of plique-a-jour enamel survive. One such object decorated in this technique is the early-15th-century Merode cup, now at the Victoria and Albert Museum. 2.4 CHAMPLEV� ENAMELINGChamplev� enameling (fig. 9, see page 253) became popular in the Middle Ages, especially for decorating the ritual vessels, crosses, and other metalwork needed for the many new cathedrals, churches, and monasteries being constructed. With the champlev� technique, enamel is applied to a less expensive metal, such as copper or one of its alloys, to create colorful accents or large areas of color. The cells for the enamel can be cast directly in the surface or be gouged or carved away from the surface of copper sheet hammered from a cast blank, as in the medieval enamels of Limoges (Biron et al. 1996). The cells are completely filled with enamel, which is finally ground level and polished. The exposed metal areas are often gilded for a richer effect. The city of Limoges became the major European center for champlev� enamel work in the 12th through the 14th centuries. In fact, in the mid-13th century the English bishops Walter de Bleys and Walter de Canteloupe state explicitly in their regulations that the Host was to be preserved in a ciborium made of silver, ivory, or “opere Limovitico” (Chamot 1930, 6). Considering this reference to the English bishops' admiration for the works of Limoges, it seems ironic that the end of the champlev� industry in Limoges was likely brought about by the sacking of that city and the massacre of many of its inhabitants in 1371 by the English Prince Edward (Verdier et al. 1977). 2.5 BASSE TAILLE ENAMELINGHistoric evidence points to Italy for the development of basse taille enameling (fig. 10, see page 254). The first piece known to have been made by the basse taille technique is the Assisi chalice commissioned by Pope Nicholas IV in 1290 (Gauthier 1970) and currently in the church of San Francesco d'Assisi. The first dated reference to basse taille enameling appears in Vasari's treatise on technique, in which he mentions that Giovanni Pisano in 1286 was commissioned to make an altar decorated with mosaics and enamels on plates of silver for the cathedral in Arezzo (Maclehose 1960). Although the basse taille technique was perfected in Italy in the 14th century, it was used extensively in England and other European countries as well. Paris became an important center for this work. In basse taille enameling the design or image is engraved or chased in low relief (intaglio) on gold or silver sheet. The cavities are then filled with brightly colored translucent enamels. Gemlike qualities are achieved as light passing through the enamel is reflected back at the viewer from the underlying lustrous metal. The enamel appears more intensely colored where the metal has been cut away or worked more deeply, since the enamel is thicker in the more recessed areas. In the final step the surfaces of the enameled areas are ground and polished. 2.6 EN RONDE BOSSE OR ENCRUSTED ENAMELINGBeginning in France in the late 14th century, 2.7 PAINTED ENAMELINGIn the mid-15th century painted enameled vessels began to appear. Painted Venetian works of that time have a distinctive appearance (fig. 12). They are made of copper covered usually with a dark blue, green, or red enamel background, decorated prominently with white areas. The surfaces are enhanced overall with gold patterns such as tiny stars or floral motifs (Lesley 1954). Also around this same time northern European Netherlandish pictorial painted enamels, such as the Walters “Ara Coeli” medallion (fig. 13, see page 254) were being produced (Verdier 1962). On this double-sided medallion, images of Emperor Augustus and the Virgin and Child are rendered in a painterly fashion in fine white enamel on a dark blue background highlighted with gold. The origin of the use of enamel to create pictorial images remains unclear. It is a matter of debate whether the earliest pictorial painted enamels were produced in Italy or the Low Countries of northern Europe. The writings of the Renaissance period reflect the competition and feelings of pride surrounding the art of enameling, making one hesitate to accept any claims of primacy at face value. From his purely Italian-centered point of reference, the 16th-century goldsmith Benvenuto Cellini wrote: Now let us have a talk about the beautiful art of
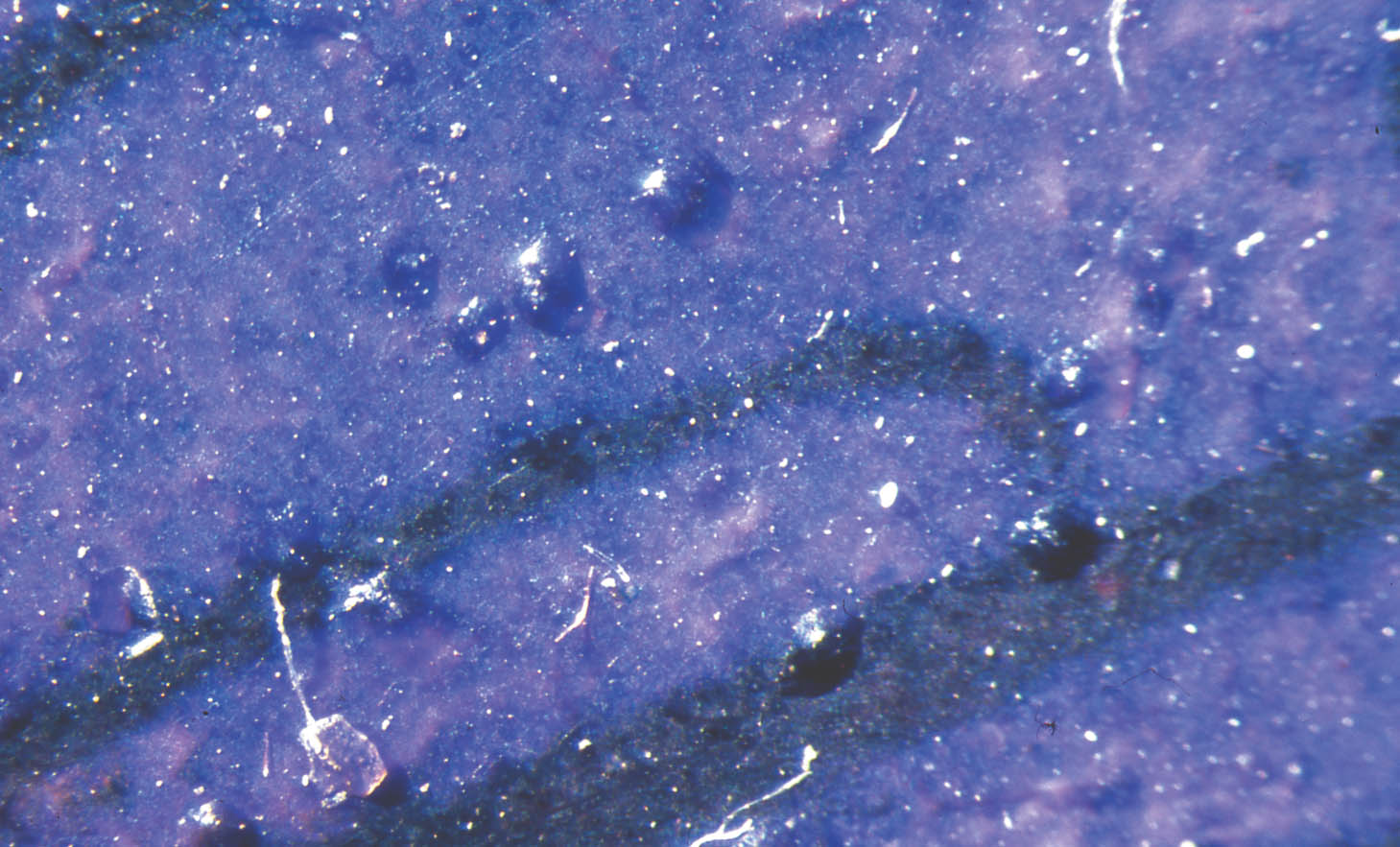
Indeed, the origins of pictorial painted enameling may never be known. Tracing influences can be especially difficult since travel and the exchange of information by goldsmiths and glassworkers were not uncommon. From English accounts we know of “Herman goldsmith” and Hans Doubler, who were not listed as freemen of the London Goldsmiths' Company and therefore were likely foreign goldsmiths. They were retained by John of Gaunt, brother of Edward III, and Richard II respectively in the late 14th century (Campbell 1997). Also, enameled objects made their way into foreign collections, such as the French enameled gifts listed in an inventory of jewelry and plate of Richard II (Campbell 1997). One gets a sense of the well-established contact between Italy and northern Europe through the writings of Antonio Neri, a Florentine priest born in 1576. His observations, L'Arte Vitraria, were published at the turn of the 17th century. Neri writes about meeting a Portuguese visitor from Antwerp named Emanuel Ximenes. He also notes that by 1601 he himself was working in a glasshouse as a craftsman. Neri traveled to Antwerp in 1603 or 1604, where he stayed until 1611. He relates that the glassmakers in Antwerp in 1535 were imitating Venetian glass and that some Venetian craftsmen were employed in an Antwerp glasshouse (Mentasti 1980). Of interest is the following commentary that shows not only the contact, but also the vain attempts to protect the local industry: On February 14, 1569, Bortolo d'Alvise presented himself at the office of the Podesta at Murano with two colleagues, with a view to denounce the masters Battista Guado and Zuan Andrea Barovier, who, with four apprentices, had had “so much audacity and temerity” as to violate the capitulary of the Guild of Glassmakers and “to leave this land and to go and practice this art in Antwerp.” (Mentasti 1980, xlvi) 3 THE EARLY PAINTED ENAMELS OF LIMOGESIn the late 15th century Limoges re-emerged as a major center for enameling, specializing in painted enamel plaques on copper. Although these enamels have certain characteristics in common with basse taille, a close examination of the Limoges enamels makes it clear how truly revolutionary they were. The Limoges enamelers broke with the tradition of incorporating the metal support into the surface design. They instead treated the entire surface of the copper support as a “canvas” or panel for painting. The images for the Limoges painted enamels of this period were mainly religious subjects based on contemporary prints. Recent research supports the belief that many Renaissance prints were handcolored soon after printing (Dackerman 2000). Perhaps these colored prints served as inspiration for the Limoges enamelers. The brilliant colors and overall painterly effects also were akin to those of small panel paintings and manuscript illuminations of the day. In fact, it is possible that the impetus for the rebirth of enameling in Limoges was to compete with the small personal devotional paintings being created at the time. Louis XI, who reigned from 1461 to 1483, restricted the right to create these painted enamels to a limited group of privileged Limoges families (Verdier et al. 1977). It is not known how many enamelers were active during this early period. Marquet de Vasselot (1921) has identified at least seven workshops judging from stylistic and technical It is unlikely that these early Limoges enamelers themselves made the glass for enameling. The glass was probably purchased in the form of ready-made cakes. We know that such stocks of enamel colors existed, since the 1326 will of Queen Maria of Hungary lists a bag containing “18 petris vitreis Rubici pro faciendis smaltis et petris vitri albi similiter” (quoted in Campbell 1997, and may be translated as “18 cakes (literally, petrais rock) of Rubicon glass for making enamel and in like manner of cakes of white glass”). Theophilus, writing in the 12th century, explained how cakes of enamel could be broken up. He recommended heating the glass until it was red hot and throwing it into a copper vessel containing water, causing the glass to splinter into small pieces ready for grinding (Dodwell 1961). The enamels were colored by the addition of metal oxides to the flux. The colorants used on the early Limoges enamels were tin for white; manganese for mulberry; copper, iron, and cobalt for blue and purple; iron and manganese for yellow; iron and copper for green (Smith et al. 1987). Amounts and ratios of colorants, firing temperature, firing time, and reducing or oxidizing conditions in the furnace atmosphere could also affect the color. Cellini provides one of the few contemporary descriptions of the enamel preparation and application process. He recommends grinding the enamel to a very fine powder in a steel mortar in very clean water and advises the enameler to make a test palette in advance to determine whether the colored enamels selected will melt in the same temperature range. He suggests applying the enamel to the copper with a brush or palette knife and describes the use of a gum (quince seed water made from soaking quince seeds in water overnight) to hold the enamel powder together during the application process. The enamel, he warns, should be heated in the furnace until the enamel just moves, but not runs. For final finishing he recommends polishing with “bits of stone or sand,” then with tripoli, or following the first polishing by putting the enamel back in the furnace and heating slowly until the enamel just begins to run (Ashbee 1967). 3.1 LIMOGES PAINTED ENAMEL TECHNIQUESA technical study of the early painted Limoges enamel plaques in the Walters collection reveals that the techniques used to create them are more complex than first meets the eye. Michaels (1968) and Smith et al. (1987) describe these techniques, which will be summarized here and supplemented with more recent observations by the author. In all cases a copper plate was used for the support. The earliest painted enamels were done on flat plates, each ranging in thickness from about 18 gauge to 28 gauge at the edges. Later, thinner, slightly domed plates came into use. The introduction of curved plates allowed the craftsman to use less metal while still providing a rigid work surface. To create the detail and modeling typical of the early Limoges images, several layers of enamel and multiple firings based on melting points were required. The upper layers had to have a lower melting point than those below to prevent remelting and disruption of the lower layers. Generally it took five or six firings. First, the copper plate was cleaned so that the enamel would adhere well. In some examples a layer of silver was applied directly to the front surface of the copper plate before the application of the enamel to create a more brilliant, reflective effect, similar to that achieved in basse taille work. Both sides of the plate had to be covered with enamel during the first firing to equalize differences in expansion and contraction of the metal and the enamel, to prevent warping from uneven heating of the plate, and to seal the metal to prevent oxidation. The enamel on the reverse, referred to as the counterenamel, was made up of the contaminated residues from the preparation of the enamels. The counterenamels of these early works tend to be thick and opaque. According to the literature, two distinctly different techniques were used to create the initial design layer on the front surface of the plaques (Gauthier and Marcheix 1962; Michaels 1968; Smith et al. 1987). The first method is the following: An initial layer of A variation on these techniques, not reported by Gauthier and Marcheix, Michaels, or Smith et al., was observed by the author during examination of a broken corner of an early Limoges enamel plaque (44.145) in the Walters collection. What can be seen in cross section are dark red lines sometimes directly on the copper plate and sometimes separated from the plate by a very thin layer of white enamel. Significantly thicker white enamel can be seen on either side of the red lines. Translucent colored enamels cover these preliminary layers. When the top surface of this enamel is viewed in raking light, one sees undulations with depressions corresponding precisely to the red drawing lines beneath. One interpretation of these observations is that first a thick layer of white enamel was applied to the front of the copper plate at the same time as the counterenamel. Before firing, the design was laid out using the enlevage technique, scratching through the white enamel and making troughlike depressions. In some cases the tool scratched through to the plate, while in others it did not quite reach the plate, leaving a thin layer of white enamel on the copper surface. The white enamel on both sides of the scratched lines retained the original thickness or perhaps became a little thicker from the displacement of the white enamel from the depressions. After firing, the design was drawn in dark red enamel in the depressions. The fact that under high magnification the red lines appear crisp and sharp and that there is no evidence of blurring or mixing of the red enamel with the white suggests that the white enamel was fired before the red lines were applied. The red lines were then fired in preparation for the application of the colored translucent enamels. This newly observed technique might represent the methods of a separate workshop or perhaps a transition between the two previously reported techniques, eliminating the need for a dark underlayer. Regardless of the technique used, the opaque white layer, once fired, provided a luminous “ground” layer, similar to the white gesso layer used in panel paintings of the day. Once the drawing of the design was complete, the plaque must have resembled a woodcut or print. It was now ready for the addition of the translucent colors. The palette was generally limited to dark blue, green, mulberry red, turquoise, and tan (light yellow brown) (Michaels 1968). Some examples also include yellow and violet, as well as the sparingly used rare and expensive clear red enamel. The powdered, colored translucent enamels were applied using the dark design lines as a guide for placement. The finely ground colored enamel frit was kept in water until ready for use. The wet enamel powder generally held together to form a paste and could be applied with a brush or palette knife. If the powder did not hold together well, a small amount of gum could be mixed in as a binder. In areas where opaque flesh tones would be added in a later step, dark translucent enamel was applied at this time to serve as a preparatory layer. Thus, the entire surface was covered at one time to a uniform level with translucent colored enamels. It is clear from the “spillover” of frit from one translucent color into another at the edges of the design areas that all of the colors were applied and fired at one time. The dark drawing lines below show through the translucent enamel, making the blurred interfaces of the colors less noticeable. An interesting phenomenon is that the color of the overlying enamel affects the appearance of the color of the drawing beneath. For example, dark red lines appear to be black when covered by blue or green translucent enamel. The surfaces of many of the early Limoges enameled plaques were enhanced with “jewels.” The creation of these embellishments was carried out during the application of the translucent enamel layer. Small squares or discs of silver foil were laid onto the unfired colored enamels, and a small The next step was to apply opaque white to selected areas of the now-fired translucent enamel, especially where flesh tones were to be represented, or details such as books, scrolls, and white articles of clothing. According to Michaels (1968), two principal methods were used for flesh tones and garments: The first employed a single layer of opaque white over a dark layer of translucent enamel. By varying the thickness of the white enamel, gradations in shades of gray could be achieved. The thinner areas appeared more gray, while the thicker areas were a purer white. Linear details, such as facial features, fingers, and garment folds, could be defined by the enlevage technique before firing, scratching through the white to the dark translucent layer beneath. In the second technique an intermediate semiopaque lilac-colored layer (a mixture of opaque white and translucent mulberry) was applied before the application of the final pure white layer. The lilac layer gave a warm tone to the white applied over it. Shading could be achieved by varying the thickness of the white layer. With this method the white areas developed a three-dimensional quality in the thickest areas. In the thinnest areas shadows were created due to the lilac tint beneath. The enlevage technique was used both through the lilac to the dark translucent layer beneath and through the pure white layer to the lilac layer to delineate fine details. It is possible that the two layers were fired separately, or, alternatively, the lilac-colored layer and the pure white could have been applied and fired at the same time. Although there are enlevage lines through the white layer to the lilac, the lines observed are not sharp and therefore could have been done while the lilac layer was dry, but not fired. After firing these opaque layers, the surface of the enamel was embellished with accents of matte, opaque red ocher for blood and wounds, and red ocher washes for tinting lips and cheeks. Dark brown was sometimes applied over areas of white for inscriptions, or lines on books, or to create other details. Gold lines, designs, and patterns further enriched the surface. These final accents were applied with a low melting point flux so that they could be fired quickly at a low temperature without disturbing the layers beneath. 3.2 DETERIORATION OF THE ENAMELSThe early painted Limoges enamels suffer from deterioration localized to particular colors. This deterioration phenomenon is further complicated by the fact that it is not always consistent, e.g., a particular color may be unstable on one enamel and stable on another, while the latter enamel exhibits instability in a color that is stable on the first. Generally, deterioration appears to be most common and consistent in the blue, mulberry, and purple areas (although other colors also may be unstable), and white areas are always stable. The damage caused by the deterioration of the enamel is significant and progressive, ranging from dulled surfaces in the initial stages to extensive loss and pitting in advanced cases. Active degradation takes the visible form of weeping (droplets of liquid forming on the surface) and crizzling (fine cracking and flaking of the enamel surface), along with the formation of white salts in some cases. The unfortunate result is that the translucency of the enamel is lost and the surfaces intended to be brilliant become dull and unattractive. It is not known when the deterioration of these enamels was first noted, but it is certainly not a new problem, as photographs taken before 1934 already record surface instability (Walters Art Museum Archives) (fig. 14). As early as 1563 a reference to deterioration in glass appears. Bernard Palissy mentions that the French glaziers blame the sun and moon for decay of their church windows (Oakley 1992). The colors that are unstable on the early Limoges painted enamels are generally stable on the enamels produced after 1530, thus indicating a
It has been shown that in the early Limoges painted enamels the blue, mulberry, and purple enamels are chemically unstable due to their original compositions (Michaels 1968; Smith et al. 1987; Biron 1999). In a study by Biron (1999) the compositions of 20 stable and unstable painted Limoges enamels were analyzed using ion beam protoninduced x-ray emission spectroscopy (PIXE) and proton-induced gamma-ray emission spectroscopy (PIGME) methods. Her findings substantially support the accuracy of the recipes for 16th-century Limoges enameling found in the writings of de Vigenere in 1615 Biron (1999). Biron writes: The enamellers then used sand or pebbles (60–65% SiO2) and as alkaline source the ash of marine plants (salicornia) from the seashores of the Provence or Spain which contained much sodium (13–15 wt.-% Na2O). However, the content of potassium (4–6% K2O) is somewhat too high to be brought into the glass by the soda ash alone. … It is possible but not certain, that potassium-rich ashes of fern or wood (beech, oak … ) were used together with salicornia.” (1999, 166) Biron found a higher concentration of potassium in the unstable blue, mulberry, and purple enamels (6–8 % potassium oxide [K2O]) than in the stable ones (4–6 % K2O), concluding that the enamelers “used a sodium source of vegetable origin for the unstable enamels as well, but added higher amounts of potassium-rich materials in comparison to the stable glasses” (1999, 167). Her analytical results also point to a different source of potassium for the stable and unstable enamels. The unstable enamels are more enriched in copper, suggesting saltpeter (potassium nitrate) as a possible source. According to Biron, de Vigenere indicated that saltpeter was recommended to create a more brilliant glass (1999). Two recipes for saltpeter appear in Biringuccio's Pirotechnia published in 1540 (Smith and Gnudi 1990) and Georgius Agricola's treatise, De Re Metallica, first published in 1556 (Hoover and Hoover 1950). These recipes specify the use of copper pots or cauldrons for boiling during the production process, and the use of sticks or rods, which may have been made of copper, for precipitating the saltpeter from solution. The use of copper in the production of saltpeter could be the source of copper enrichment in the unstable enamels. Michaels concluded that excessive amounts of sodium caused the instability of the enamels at the Walters. He also reported the results of x-ray diffraction analysis on the white surface salts, which were identified as sodium formate, possibly from a reaction of the sodium with formic acid off-gassing from display and storage materials (1968, 35). However, for the objects she examined, Biron identified higher levels of potassium as the primary factor causing the depolymerization of the glass structure, making the enamels more prone to be affected by the environment (1999, 168). Smith et al. (1987, 109) also identified In addition to Michaels (1968), Smith et al. (1987), and Biron (1999), a number of other articles have appeared on the degradation of early painted Limoges enamels (Philippon et al. 1988; Eveno et al. 1990; Perez y Jorba et al. 1993; Germain-Bonne et al. 1996; Biron and Belassene 1999). A review of literature on crizzling and weeping vessel glass also shows agreement about the causes of deterioration. It is generally accepted that the degradation process involves the interaction of the glass layer with atmospheric moisture, causing hydration of the surface (Brill 1972). In summary, the alkali ions, such as sodium and potassium, tend to migrate to the glass surface where an exchange takes place with water molecules and/or hydronium ions (H3O+). In unstable glass, alkali substitution may occur at the same time as network dissolution, “forming a silica-rich hydrated layer having a more open structure through which ions and molecules can easily diffuse”(Ryan et al. 1996, 842). When the hydrated layer on the surface dehydrates, shrinkage occurs, causing cracks (Hogg et al. 1998). In addition, alkali salt deposits can form. Due to the deliquescent nature of the alkali-enriched surface, a solution with a pH above 9 is created, which can accelerate attack on the silica network of the glass. This attack can open the glass or enamel surface to continued deterioration (Ryan et al. 1996). The author has noted an additional problem for the enamels that does not affect vessel glass: the alkaline liquid formed during deliquescence can seep through cracks in the enamel to the underlying copper plate, resulting in separation of the enamel from the plate as corrosion products form on the copper (fig. 15, see page 255). The deterioration of the early painted Limoges enamels can be particularly disheartening to those involved in their care.
Since the cause of the deterioration is inherent in the enamel itself, there may be no way to completely “cure” the problem. It has been suggested that lowering the relative humidity to prevent surface hydration may protect the enamels (Smith et al. 1987); however, lowering the relative humidity tends to increase crizzling and the rate of surface loss from flaking on the glass surface (Brill 1975). Although controlling the environment may slow the reactions involved in the deterioration, it does not present a permanent solution as even minor fluctuations in relative humidity can cause the enamel to cycle between weeping and crizzling. In an experiment in 1986, the author witnessed weeping on enamels above 52% RH and crizzling below 48% at 71�F. It should be noted that the Limoges enamels at the Walters were kept in an air-conditioned, thick-walled, well-insulated building where the environment was always fairly well controlled, usually between 47% and 53% RH and 68� and 72�F. 4 THE WALTERS COLLECTION OF EARLY PAINTED LIMOGES ENAMELSAccording to Philippe Verdier in his catalogue of the painted enamels of the Renaissance in the Walters Art Gallery, “From the 1830s and 1840s, no French art collector in the field of European decorative arts considered his collection complete without including masterpieces of the Limoges enamellers” (1967, ix). These enamels were also highly sought after by the English. Until World War I these enamels brought very high prices on the art market. Verdier continues: “Limoges enamels were looked upon by connoisseurs as supreme embodiments of form and color and as perfect fusions of draughtsmanship and the art of the goldsmith” (1967, ix). According to Verdier, the collection of painted Limoges enamels in the Walters:
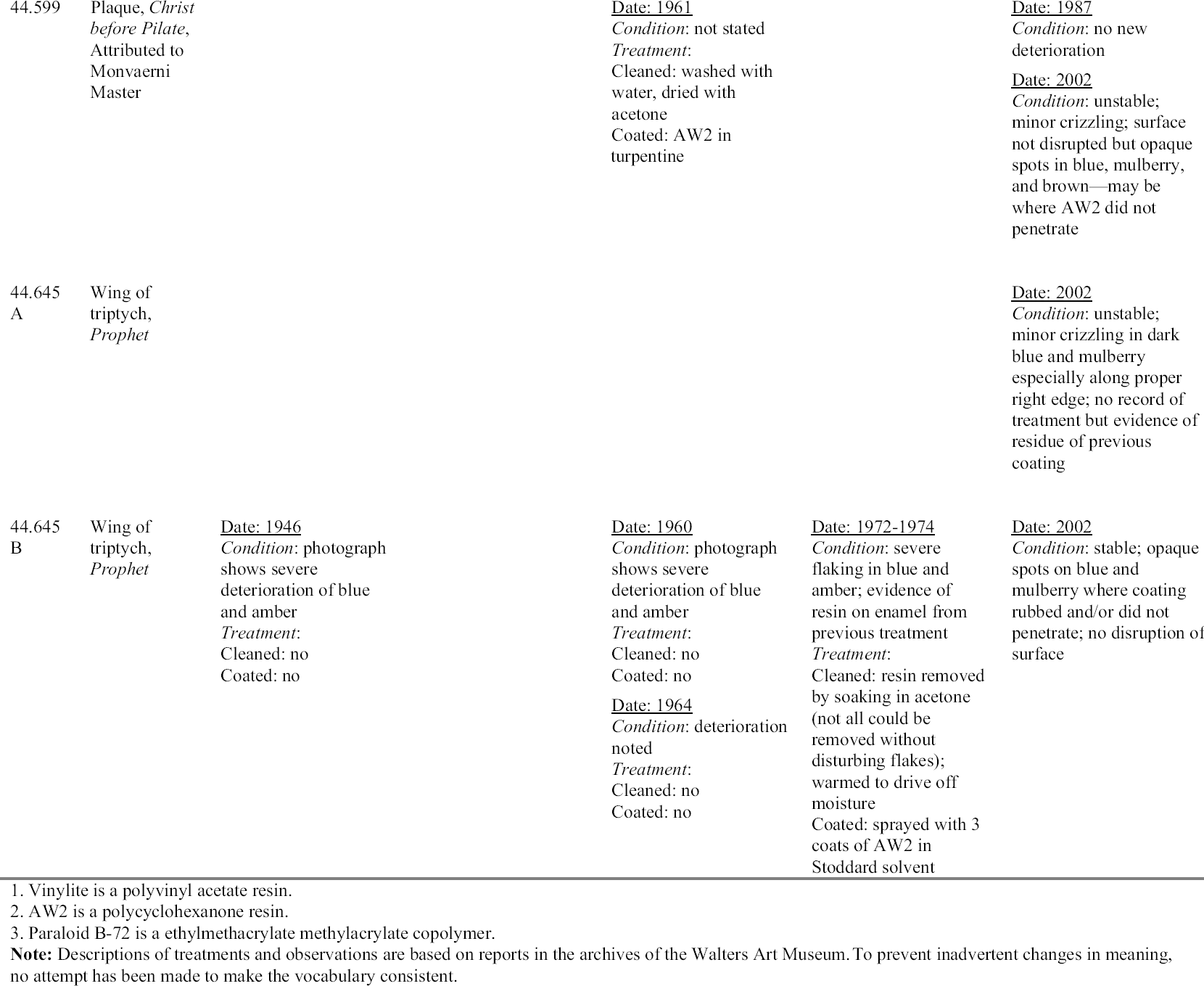
Of the 201 painted Limoges enamels in the Walters collection cataloged by Verdier, 25 were identified as being made between 1470 and 1530, the dates recognized as the beginning and end of the early painted Limoges enamel period. Most of the enamels were acquired by Henry Walters in the early 20th century through various dealers. If Verdier is correct about the values placed on these enamels up to World War I, Henry Walters bought at the peak of the market. His collection today continues to amaze and delight the public and scholars, as many of the enamels retain their jewel-like character. For this reason it is especially important to inhibit further deterioration of the collection for the enjoyment of future generations. 4.1 HISTORY OF DETERIORATION AND TREATMENTIn the 1960s Peter Michaels, a painting conservator at the Walters, noted that the early painted Limoges enamels in the collection had deteriorated and showed evidence of cracking, spalling surfaces, and white salt efflorescence. The salts were identified by x-ray diffraction analysis and found to be sodium formate. As mentioned above, the source of the sodium was the alkali leaching from the enamel, and the formate was thought to be from contaminants in the environment. Another possible source of the formic acid not mentioned by Michaels was offgassing from the oak frames used to remount many of the enamels in modern times. Michaels began a treatment campaign to stabilize the actively deteriorating enamels. According to the records in the files, the premise for his treatments was to remove the salts and to consolidate the surfaces of the enamels to improve their appearance and to prevent further loss of detached flakes. The early treatment records indicate that many of the unstable enamels Michaels was to treat had been powdery and deteriorated in 1940 and had been coated at that time with Vinylite, an early polyvinyl acetate resin. (The records do not indicate what molecular weight Vinylite was used.) This previous attempt to stabilize the enamels had been unsuccessful, and in 1960 the enamels were in an even more advanced state of deterioration. Michaels's treatments began with cleaning the enamels to remove the decomposing Vinylite coating. He was not consistent in his choice of solvents for this task and seems to have used singly and in combination alcohol, water, benzine, acetone, and toluene. A variety of solvents was used because he found the Vinylite and other coatings that were not identified to be difficult to remove. Similar problems of coating removal on enamel have recently been noted by Magee (1999) after attempts to remove polyvinyl acetate (AYAA) from a crizzling enamel tazza in the collection of the Art Institute of Chicago. Besides using solvents to remove the coating, Michaels used water to dissolve salts, and alcohol and acetone to dry the enamels before applying a new coating. His records do not always state what type of alcohol or whether the water he used was distilled or from the tap. Although some treatments indicate that he swabbed the surfaces with water, others are not clear. Two treatments carried out in 1963 specifically mention immersing the enamels in acetone, then water, then alcohol. Michaels's treatments, as well as other treatments carried out on this group of enamels, are summarized in appendix 1.
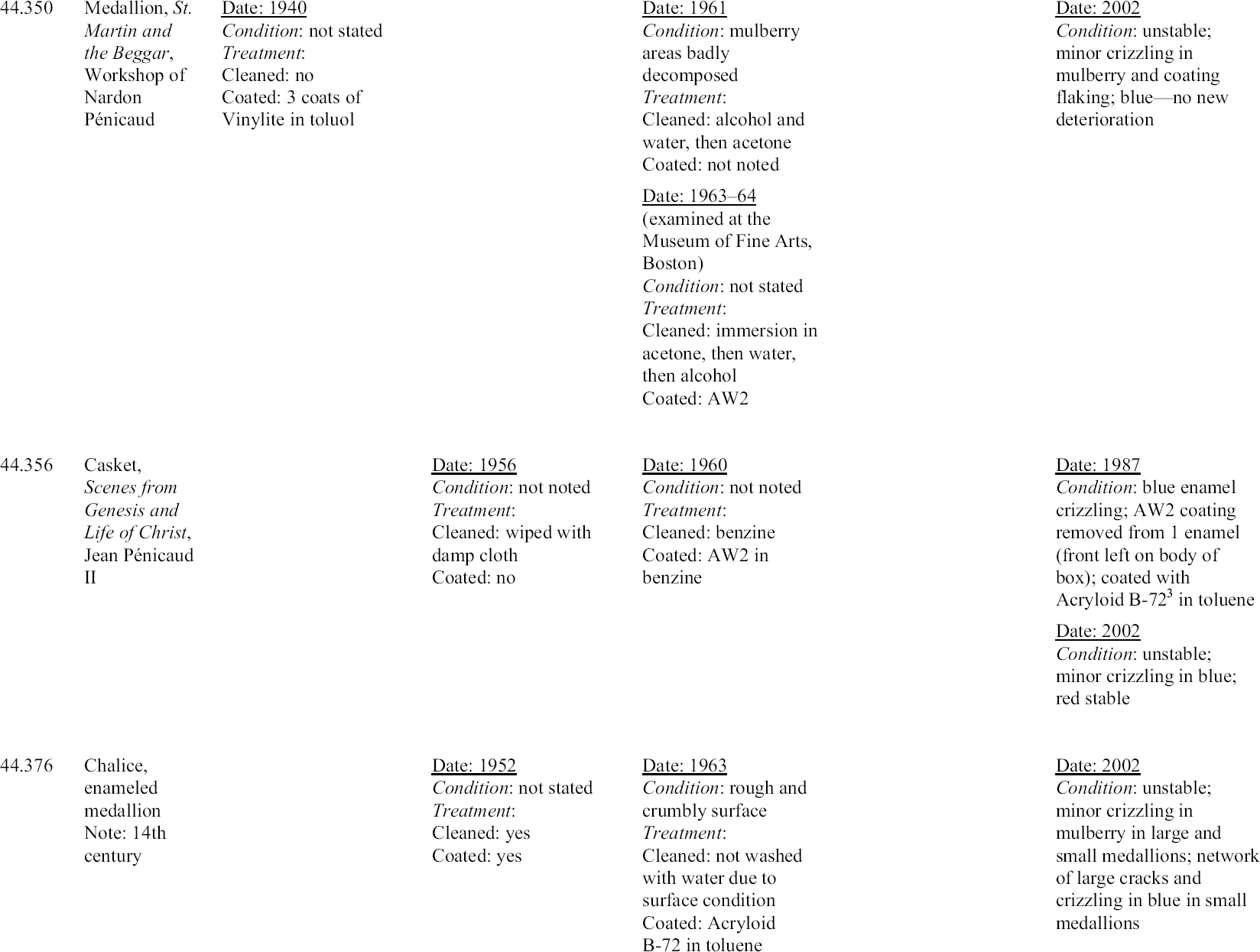
Michaels (1968) described his treatment of enamel 44.126 (Virgin and Child with Parrot) (figs. 16, 17). He indicated that he submerged the enamel in water to remove the soluble alkaline salts, did a final rinse in distilled water, and put the plaque through two changes of ethyl alcohol, and finally acetone, to remove all of the water. After a thorough airing, he coated the surface heavily with AW2 (a ketone resin, polycyclohexanone) in benzine. It is not clear from the in-house records that Michaels did, in fact, submerge that particular plaque. According to documents in the files, the enamel was cleaned with alcohol and water, then acetone, and was rinsed in acetone, then methyl alcohol, then acetone. Michaels
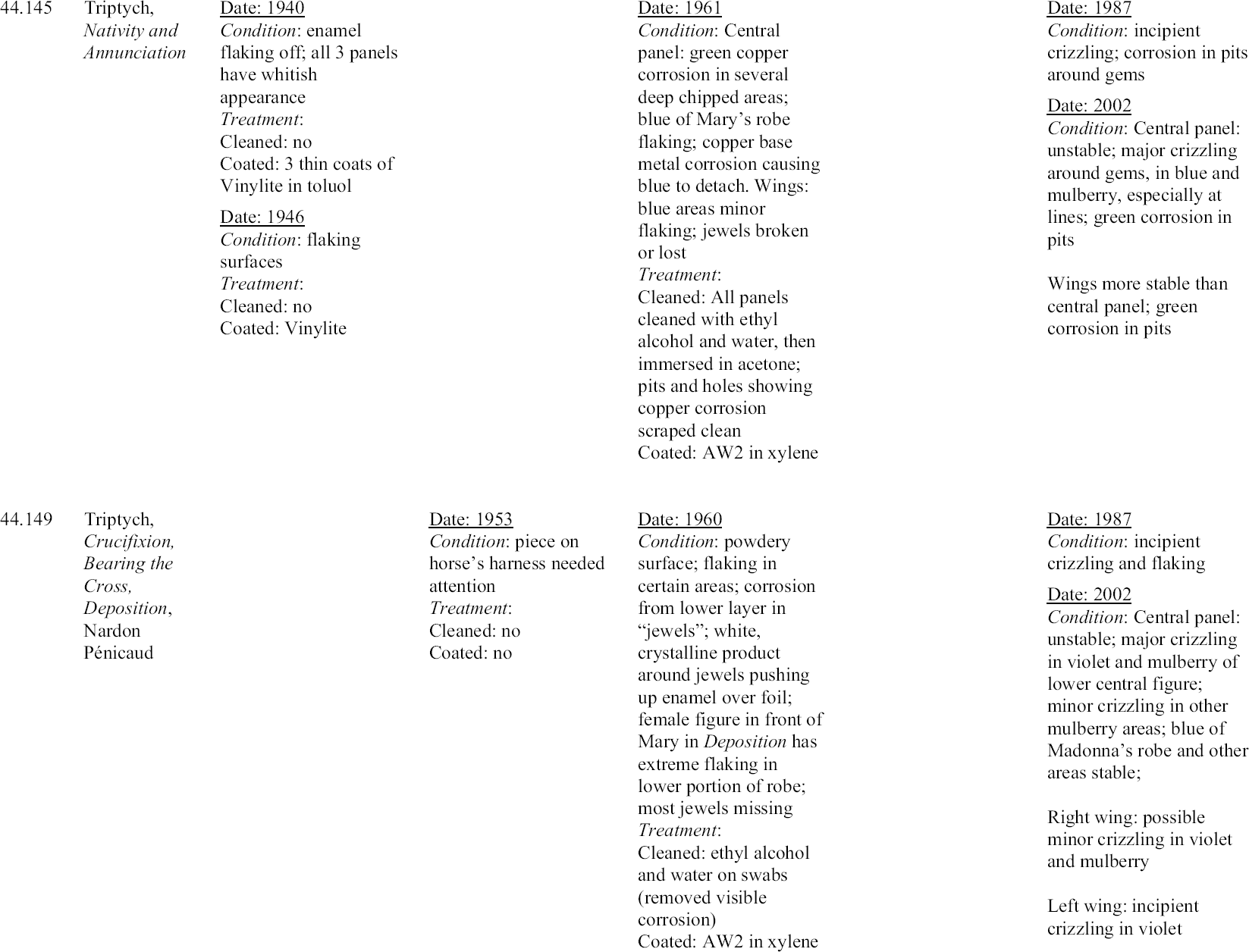
Michaels selected AW2 resin for coating even though he did not consider it a superior moisture barrier. He felt it was readily soluble and that it would penetrate well into the flaking surfaces. He surmised that the thickness of the coating would compensate for its lower resistance to moisture and felt that the sheen of the resin approximated that of the enamel (1968). Although Magee reports (1999) that the AW2 resin on the Walters enamels has become insoluble, this statement is incorrect and likely the result of a misunderstanding. The resin remains soluble, but sometimes requires the use of more polar solvents and longer exposure to solvents for removal. These observations are supported by solubility tests carried out by Feller and Curran (1975) on aged ketone resin films. In 1970 an enamel triptych (44.316) (fig. 18) that had been treated by Michaels 10 years earlier was brought to the conservation laboratory. The central panel was an image of the Annunciation, and each wing was a prophet. The enamel was attributed to the Master of the Orl�ans Triptych. The history of the treatment of this enamel was unclear before Michaels's treatment. A 1936 photograph showed deterioration in the blue areas, but no treatment had been recorded. In 1953 there is some indication that it was coated with Vinylite. In 1960, when it was examined by Michaels, almost all of the blue was crazed and deteriorated (fig. 19). The surface was powdery, and there were small, detached chips. There was a white powdery substance on the blue surfaces, and copper salts from the plate were pushing off the enamel. Michaels treated the enamel by removing the corrosion mechanically and with acetone, alcohol, and benzine. He found a film on the surface, but determined that it did not dissolve in toluene, acetone, or alcohol. He removed this film with alcohol
An examination of this enamel in 1970 showed that there was new green corrosion on the reverse from the interaction of the copper with the alkaline products forming on the enamel. It was also evident that the crizzling had continued. At this time the author was assigned the treatment of the object. First the green corrosion products were removed mechanically. Next the AW2 resin was removed in baths of Stoddard solvent, benzine, then Stoddard solvent and xylene. Based on Michaels's treatment as represented in his 1968 article, as well as advice from Elisabeth Packard, then director of the Walters Conservation Laboratory, the enamel was immersed in four successive 20-minute baths of distilled water to remove the salts. It was then immersed in five successive baths of acetone at 10-minute intervals to remove the water. After airing overnight, the enamel was warmed slightly in a case with heat from a light bulb to drive off any excess moisture. Finally, the surface of the enamel was sprayed with six coats of 36% AW2 resin in Stoddard solvent, allowing it to dry and warming slightly between coats. The translucency was restored to the enamel, and it has not changed since that time (fig. 20). Another treatment was carried out by the author on an enamel plaque (44.645B) in 1972. The enamel had not been treated by Michaels, but microscopic examination revealed a coating that seemed similar in appearance to other Vinylite coatings. The enamel was flaking severely in the blue and amber areas. The coating was tested for solubility and found to be difficult to remove. Acetone appeared to work the best, but it did not remove the coating completely, and residues of the resin remained in the cracks. The enamel was then warmed slightly in a case with heat from a light bulb, and finally sprayed with three coats of 36% AW2 resin in Stoddard solvent. Two enamels also were coated with Paraloid B-72 (formerly Acryloid B-72: ethylmethacrylate
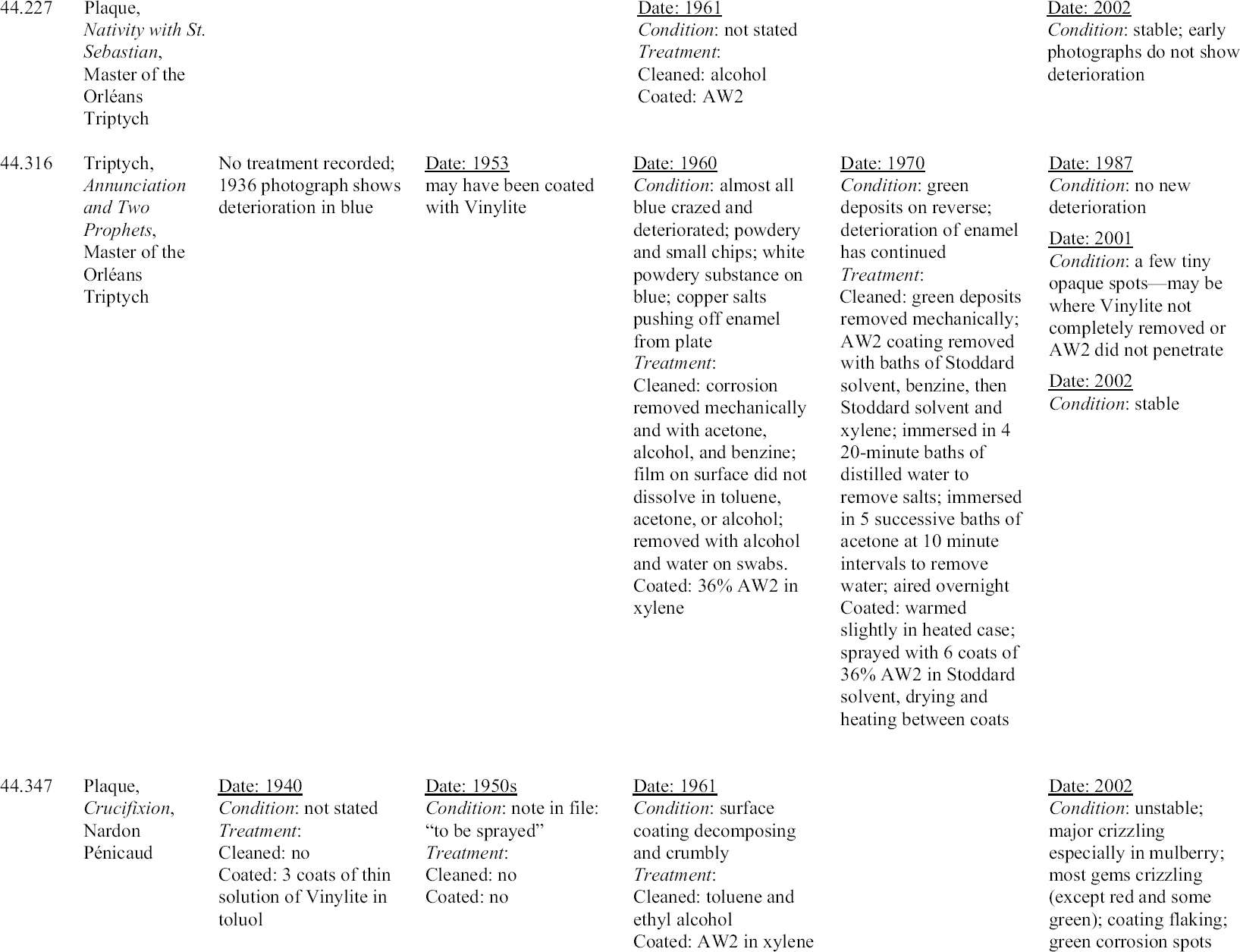
4.2 EVALUATION OF PAST TREATMENTSForty years have elapsed since Michaels's treatments were carried out, and 30 years since the retreatment of enamels by the author. Although a lack of detail in the records makes evaluation imprecise, visual examination through a microscope can give some sense of the success of the previous treatments. Since the treatment of unstable Limoges enamels is still a challenge for the conservator, any information one can glean about the long-term behavior of materials and treatment regimens used with these objects is of value. Appendix 1 summarizes the treatment history of each enamel based on the conservation records. To prevent inadvertent changes in meaning, descriptions of condition and treatment in appendix 1 have been reported as in the original, and no attempt has been made to make the vocabulary consistent. The column on the far right in appendix 1 indicates the current condition of the enamel evaluated with a binocular microscope at 25x. For the purposes of this study, an enamel was considered unstable if any area, no matter how small, was found to be weeping or disrupted by crizzling. Three categories of crizzling were established based on the level of activity. These were identified in the table as minor A review of the results of Michaels's treatments may initially indicate a lack of success. Most of the enamels have exhibited some degree of ongoing or current deterioration. However, in every case, the condition is considerably better than it was before Michaels's treatments in 1960. In fact, the new activity is generally localized and in many cases difficult to see without the aid of magnification. A comparison of photographs of before and after treatment in 1960 and today's condition makes this point clear (figs. 21–27). One enamel that was treated but initially does not seem improved is 44.128. This plaque was cleaned and coated in 1961. Then in 1963, after removing the coating, it was soaked in water, but not recoated. The surface is currently crizzling overall and is efflorescing with white salts. However, if one compares its current condition with its condition in photographs taken before treatment in 1960 and during treatment in 1961, it is not clear that its condition has worsened. It appears that the enamel is in better condition than before treatment in 1960, and in similar condition to that in 1961 after cleaning but before coating. Of interest is the fact that no new deterioration is evident on enamel 44.316, which had had a history of recurring deterioration and had been re-treated by the author in 1970 (fig. 28, see page 255). During retreatment this enamel was more thoroughly soaked in distilled water than the others, and it is likely that the soluble elements of the glass, usually involved in the deterioration process, were removed from deeper within the enamel layer. This procedure may have left the upper layer of the glass less reactive with the environment. Since the enamel was heated slightly during drying, excess moisture was driven off just before coating. This step allowed the resin to flow more readily into the cracks, creating a better barrier than
The enamel (44.645B) treated by the author in 1972 also has remained stable. This enamel was not soaked in water, but was warmed before three spray coats of AW2 resin were applied. Therefore, this finding may support the possibility that warming and/or thickness of the coating plays a significant role in preservation. The enamels coated with Paraloid B-72 have not remained stable. Since only one coat of a relatively dilute solution of Paraloid B-72 was applied, more study should be carried out applying thicker or more concentrated coatings of this resin. A comparison of the current condition of the treated enamels to two enamels that have never been treated (44.438 and 44.136) is compelling and informative (figs. 29, 30; see also figs. 2, 3, and 5). The untreated enamels are in far worse condition than all of the treated enamels. A comparison of photographs taken in 1977 and 2002 showing details of the collar on a figure in untreated enamel 44.438 documents a significant progression of disruption and loss (figs. 31, 32, see page 256). Generally there is severe crizzling on the untreated enamels with total detachment of flakes from the surface. The blue and mulberry enamels on 44.438 have been observed on many occasions cycling between crizzling and weeping (see fig. 3, page 252). The enamel on this piece is being pushed from the substrate by corrosion caused by a reaction of the copper plate with the alkaline liquid that forms during weeping. It is clear that the untreated enamels have suffered more surface loss and are currently more active than the enamels that have been treated. 4.3 OBSERVATIONSThe following observations can be made after reviewing the treatment records, photographic documentation, and current condition of 25 early painted Limoges enamels in the Walters collection:
Although it is tempting to conclude from these observations that washing, drying, warming, and thickly coating the actively deteriorating painted enamels is a satisfactory treatment, it must be kept in mind that there are many risks involved. Washing with water can remove salts, but it also can alter the chemical makeup of the enamel. In this instance such alteration may have its long-term advantages for stability; however, the introduction of water to the enamel can also encourage further hydration of the enamel surface and activation of corrosion of the copper plate. Immersing the enamel in solvents such as ethanol and acetone to dry them can cause shrinkage of the hydrated layer and encourage crizzling and cracking. Also, the lowering of the temperature of the enamel with acetone can cause condensation to occur immediately after drying, again introducing water into the enamel. Warming the enamel is generally not advisable, as crizzling can be encouraged during drying. Coating enamels can have positive and negative aspects. Coatings may give some protection from atmospheric moisture and can secure flaking surfaces and restore translucency by filling in cracks. As has been demonstrated, however, a coating can cause problems as well. While the AW2 resin used on the Walters enamels remains soluble, has not visibly yellowed, and has held up well over time, the Vinylite resin broke down and became significantly less soluble relatively quickly. Deterioration continued beneath the Vinylite, and enamel spalled away with the detaching coating. When the same enamels were later coated with AW2 resin, they underwent significantly less damage, even when crizzling recurred. It is possible that the AW2 resin performed better due to a combination of factors. The resin's ability to penetrate tiny cracks in the enamel surfaces easily and the fact that the resin was applied in a thick layer may have inhibited the interaction of the enamel with the
4.4 THE FUTURE OF EARLY PAINTED LIMOGES ENAMELSOne must weigh all the potential risks discussed above, but while waiting for the development of a treatment or environment that will preserve the enamels, active deterioration inevitably proceeds. For those who must care for early painted enamels from Limoges, this dilemma is especially profound. The evaluation of the current condition of the Walters collection of early painted Limoges enamels can give us some basis for future directions in approaching the preservation of these objects. The inherent instability of these objects is a major challenge, and much still needs to be done even to determine the best environment for their storage and display. Analysis of the enamels currently being carried out by French, German, and American scientists may lead to a better understanding of the causes of deterioration and how treatments may impact the enamels themselves. The results of these studies were reported at the International Symposium on Limoges Painted Enamels held in 2002 in Braunschweig, Germany, and should be published in the future. The treatments carried out at the Walters, even though successful to varying degrees, need further study, and other treatments need to be developed and evaluated. There is some promise for at least a better understanding of the mechanisms involved in the deterioration processes and long-term evaluation of possible treatments and coatings. Discussions have recently taken place among scientists and several conservators in institutions with collections of unstable enamels. It is through such collaboration that the early painted enamels of Limoges finally may be saved. ACKNOWLEDGEMENTSI wish to thank my colleagues at the Walters for their support and forbearance as I covered every surface in the laboratory with Limoges enamels and monopolized microscope and cameras on many occasions. I am especially indebted to Meg Craft, Julie Lauffenburger, and Robert Weisser, who willingly and with the best of humor read too many drafts of this article. They are responsible for many improvements to the text. I also wish to thank Dawn Brown, who converted my nearly illegible notes into appendix 1. NOTES1. The author has seen evidence of similar surface loss or active deterioration in collections at the Metropolitan Museum of Art, the Frick Collection, the Victoria and Albert Museum, the Taft Museum, the Herzog Anton Ulrich Museum, and the Louvre. Photographs of Limoges enamels from other collections also suggest that this type of damage is widespread in early painted Limoges enamels. APPENDIX
REFERENCESAshbee, C. R., trans. 1967. The treatises of Benvenuto Cellini on goldsmithing and sculpture. New York: Dover Publications. Barsali, I. B.1969. European enamels. London: Paul Hamlyn. Biron, I.1999. Study of XVth and XVIth century painted enamels through scientific analysis: Causes of glass deterioration. Berliner Beitr�ge zur Arch�ometrie16:163–74. Biron, I., and M.Belassene. 1999. Reproduction de l'alt�ration des �maux peints. ICOM Committee for Conservation preprints, 12th Triennial Meeting, Lyons. London: ICOM. 764–69. Biron, I., P.Dandridge, and M. T.Wypyski. 1996. Techniques and materials in Limoges enamels. In Enamels of Limoges, 1100–1350.New York: Metropolitan Museum of Art. 48–62. Brill, R.1972. Incipient crizzling in some early glasses. Bulletin of the American Group—International Institute for Conservation of Historic and Artistic Works12(2):46–47. Brill, R.1975. Crizzling: A problem in glass conservation. In Conservation in archaeology and the applied arts.London: IIC. 121–34. Campbell, M.1997. English basse taille enamels. Annali della scuola normale superiore di pisa4(2):37–46. Caroselli, S. L.1993. The painted enamels of Limoges. Los Angeles: Los Angeles County Museum of Art. Chamot, M.1930. English mediaeval enamels. London: Ernest Benn. Dackerman, S.2000. Painted prints. University Park: Pennsylvania State University Press. Dodwell, C. R., trans. 1961. Theophilus: The various arts. London: Thomas Nelson and Sons. Eveno, M., D.Fromageot, B.Kusko, and C.Lahanier. 1990. �tude de l'alt�ration d'use plaque �maill�e attribu�e � la production de Limoges. ICOM Committee for Conservation preprints,9th Triennial Meeting, Dresden. Los Angeles: ICOM. 18–23. Feller, R., and M.Curran. 1975. Changes in solubility and removability of varnish resins with age. Bulletin American Institute for Conservation15(2):17–26. Freestone, I. C.1992. Theophilus and the composition of medieval glass. In Materials issues in art and archaeology III, ed. P.Vandiver et al. Pittsburgh: Materials Research Society. 739–45. Gauthier, M.1970. Les �maux du moyen �ge. Fribourg: Office du Livre. Gauthier, M., and M.Marcheix. 1962. Limoges enamels. London: Paul Hamlyn. GermainBonne, D. I.Biron, and P.Trocellier. 1996. La d�gradation des �maux peints des XV et XVI si�cles. ICOM Committee for Conservation preprints,11th Triennial Meeting, Edinburgh. London: ICOM. 826–32. Hogg, S. E. T., D. S.McPhail, P. S.Rogers, and V. L.Oakley. 1998. Mono-functional organosilanes as candidates for treatments of crizzling in glasses. In Glass, ceramics, and related materials, ed. A. B.Paterakis. Vantaa, Finland: EVTEK Institute of Arts and Design. 53–57. Hoover, H. C., and L. H.Hoover, trans. 1950. Georgius Agricola De re metallica. New York: Dover Publications. Lesley, E. P., Jr.1954. Enamel: An historic survey to the present day. New York: Cooper Union Museum for the Arts of Decoration. Maclehose, L. S., trans. 1960. Vasari on technique. New York: Dover Publications. Magee, C. E.1999. The treatment of a severely deteriorated enamel. ICOM Committee for Conservation preprints, 12th Triennial Meeting, Lyons. London: ICOM. 787–92. Marquet de Vasselot, J. J.1921. Les �maux Limousins de la fin du XVe si�cle et de la premi�re partie du XVIe. Paris: Auguste Picard. Maryon, H.1971. Metalwork and enamelling.5th ed.New York: Dover Publications.
Mentasti, R. B., trans. 1980. Antonio Neri L'arte vetraria. Milan: Edizioni il Polifilo. Michaels, P. E.1968. Technical observations on early painted enamels from Limoges: Their materials, structure, technique, and deterioration. Journal of the Walters Art Gallery27–28:21–43. Oakley, V.1992. The deterioration of vessel glass. In Glass and enamel conservation. UKIC occasional papers 11. London: UKIC. 18–22. PerezyJorba, M. M.Rommeluere, and L.Mazerolles. 1993. �tude de la d�terioration d'une plaque d'email peint de Limoges. Studies in Conservation38(3):206–12. Philippon, J., G.Bossiere, and B.Beillard. 1988. Examen par microscopie �lectronique � balayage des alt�rations d'�maux peints du XVI siecle. In Glass studies.2d International Conference on Nondestructive Testing, Microanalytical Methods and Environment Evaluation for Study and Conservation of Works of Art. Rome: Instituto Centrale Per Il Restauro—Associazione Italiana Prove Non Distruttive. 8. 1–8. 11. Ross, M.1941. Bassetaille enameling at Montpellier. Art Quarterly4(1):32–39. Ryan, J. L., D. S.McPhail, P. S.Rogers, and V. L.Oakley. 1996. Glass deterioration in the museum environment: A study of the mechanisms of decay using secondary ion mass spectrometry. ICOM Committee for Conservation preprints,11th Triennial Meeting, Edinburgh. London: ICOM. 839–44. Smith, C. S., and M. T.Gnudi, trans. and eds. 1990. The pirotechnia of Vannoccio Biringuccio. New York: Dover Publications. Smith, R., J. H.Carlson, and R. M.Newman. 1987. An investigation into the deterioration of painted Limoges enamelplaques, c.1470–1530. Studies in Conservation32:102–13. Verdier, P.1957. An enamelled triptych by the Master of the Orl�ans Triptych. Bulletin of the John Herron Art Institute44(3):42–46. Verdier, P.1962. A medallion of the “Ara Coeli” and the Netherlandish enamels of the fifteenth century. Journal of the Walters Art Gallery24:9–37. Verdier, P.1967. Catalogue of the painted enamels of the Renaissance. Baltimore: Trustees of the Walters Art Gallery. Verdier, P., M. S.Dimand, and K. C.Buhler. 1977. Enamels, rugs, and silver in the Frick Collection. New York: Frick Collection. Walters Art Museum Archives, Baltimore. AUTHOR INFORMATIONTERRY DRAYMAN-WEISSER has been associated with the Walters Art Museum for more than 30 years and has been director of conservation and technical research since 1977. After apprenticing with Elisabeth Packard at the Walters, she received her conservation training in objects at the Institute of Archaeology, University of London, graduating in 1973 with distinction. She has published widely on diverse subjects, such as archaeological copper alloys, ivory, and conservation training. Her special interests also include jewelry and enamels. She was a contributing author to Caring for Your Collections (1992), and has recently edited the book, Gilded Metals (2000). After serving AIC in various capacities, including as president, she is currently chair of the AIC Certification Development Committee.
 Section Index Section Index |