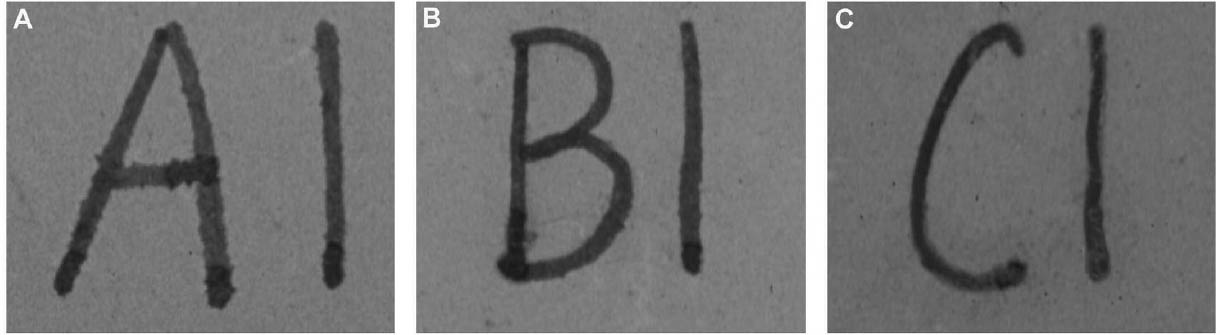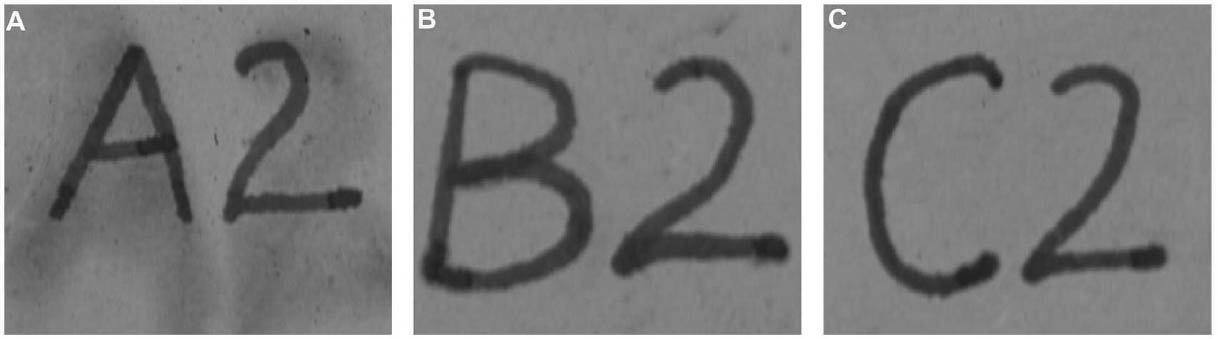THE USE OF CYCLODODECANE AS A TEMPORARY BARRIER FOR WATER-SENSITIVE INK ON ARCHAEOLOGICAL CERAMICS DURING DESALINATIONVANESSA MUROS, & JOHN HIRX
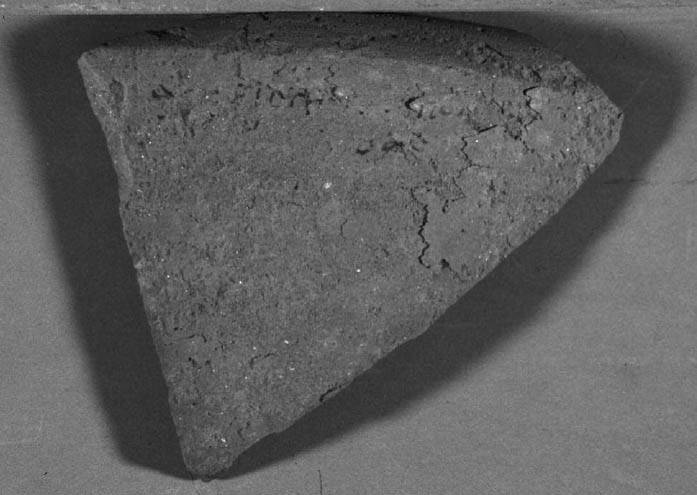
ABSTRACT—This article discusses a preliminary study into the use of cyclododecane as a temporary barrier for the desalination of inscribed archaeological ceramics. Prior to desalination, pigmented or water-sensitive areas on ceramic objects are often consolidated with a resin. This process may alter the appearance of the object even after the resin has been removed. Cyclododecane, a volatile binder, has been used in the field of paper conservation for temporarily fixing water-sensitive media during aqueous treatments. This technique was adapted to conduct tests to determine whether water-sensitive media on archaeological materials could be temporarily protected with cyclododecane during desalination. Several marked modern terracotta samples were coated with cyclododecane using various application methods, and the samples were immersed or poulticed in tests simulating desalination. With the results obtained from these tests, an inscription on an Egyptian ostracon was coated with cyclododecane and desalinated. The results of the experiments and case study show that cyclododecane, when applied as a melt, offers considerable protection for water-sensitive ink during the desalination of archaeological ceramics and is a viable alternative to other materials commonly used for this purpose. TITRE—L'utilisation du cyclodod�cane en tant que fixatif temporaire pour des encres solubles � l'eau lors du dessalement des c�ramiques arch�ologiques. R�SUM�—Cet article pr�sente une �tude pr�liminaire sur l'utilisation du cyclodod�cane comme fixatif temporaire lors du dessalement de c�ramiques arch�ologiques avec dessins ou inscriptions. Pr�alablement au dessalement, la surface des c�ramiques sensibles � l'eau ou couvertes de pigments est fr�quemment consolid�e � l'aide d'une r�sine. Cette fa�on de faire risque d'alt�rer l'apparence de l'objet, m�me apr�s que la r�sine ait �t� enlev�e. Le cyclodod�cane, un liant volatile, a �t� utilis� dans le domaine de la restauration des œuvres sur papier comme fixatif temporaire lors du traitement des m�dias sensibles � l'eau. Cette technique a �t� adapt�e afin d'effectuer des tests permettant de d�terminer si des m�dias sensibles � l'eau pouvaient �tre temporairement prot�g�s avec le cyclodod�cane lors du dessalement. Plusieurs fragments de terre cuite portant des inscriptions furent enduits avec du cyclodod�cane � l'aide de m�thodes d'application vari�es, et les �chantillons furent immerg�s ou trait�s � l'aide d'un cataplasme lors de tests simulant un dessalement. � l'aide des r�sultats provenant de ces tests, une inscription sur un ostracon �gyptien fut recouverte avec du cyclodod�cane et l'objet ensuite dessal�. Les r�sultats de ces exp�riences et l'�tude de cas d�montrent que le cyclodod�cane, lorsqu'il est d'abord fondu et appliqu� sur la surface, offre une tr�s bonne protection pour les encres sensibles � l'eau lors du dessalement des c�ramiques arch�ologiques et constitue une alternative int�ressante aux autres produits utilis�s � cette fin. TITULO—Estudio preliminar del uso del ciclododecano como barrera temporal de tintas sensibles al agua durante la desalinizaci�n de cer�micas. RESUMEN—Este art�culo presenta un estudio preliminar del uso de ciclododecano como barrera temporal para la desalinizaci�n de cer�micas arqueol�gicas con inscripciones. Previo a la desalinizaci�n, los objetos de cer�mica con �reas pigmentadas o sensibles al agua son, por lo general, consolidadas con resina. Este proceso puede alterar la apariencia del objeto, a�n despu�s de que la resina ha sido removida. El ciclododecano, un aglutinante vol�til, ha sido utilizado en la conservaci�n de papel para la fijaci�n temporal de medios sensibles al agua durante tratamientos acuosos. Esta t�cnica fue adaptada para conducir pruebas y determinar si los medios sensibles al agua en materiales arqueol�gicos pueden ser temporalmente protegidos con ciclododecano durante la desalinizaci�n. Varias muestras de terracota moderna fueron recubiertas con ciclododecano utilizando distintos m�todos de aplicaci�n, y se sumergieron o se le aplicaron compresas para simular tratamientos de T�TULO—O uso de ciclododecano como prote��o tempor�ria para tintas sens�veis � �gua durante a dessaliniza��o de cer�micas arqueol�gicas. RESUMO—Este artigo examina um estudo preliminar sobre o uso de ciclododecano como prote��o tempor�ria durante o processo de dessaliniza��o de cer�micas arqueol�gicas com inscri��es. Antes da dessaliniza��o, as �reas pigmentadas ou sens�veis � �gua nos objectos cer�micos s�o geralmente consolidadas com uma resina. Este processo pode alterar a apar�ncia do objecto mesmo depois da resina ter sido removida. Ciclododecano, uma liga vol�til, tem sido utilizado no campo da conserva��o de papel para fixar temporariamente os meios sens�veis � �gua durante os tratamentos aquosos. Esta t�cnica foi adaptada para a realiza��o de testes a fim de determinar se os meios sens�veis � �gua em materiais arqueol�gicos poderiam ser temporariamente protegidos com ciclododecano durante a dessaliniza��o. V�rias amostras modernas de terracota marcada foram revestidas com ciclododecano usando distintos m�todos de aplica��o, e as amostras foram submersas ou foi-lhes aplicado um cataplasma, em testes simulando a dessaliniza��o. Com os resultados obtidos nestes testes, uma inscri��o num ostrac�o eg�pcio foi revestida com ciclododecano e dessalinizada. Os resultados das experi�ncias e estudo de casos mostraram que o ciclododecano quando aplicado em estado derretido, oferece uma protec��o consider�vel para as tintas sens�veis � �gua durante a dessaliniza��o de cer�micas arqueol�gicas e � uma alternativa vi�vel em rela��o a outros materiais utilizados habitualmente com este objetivo. 1 INTRODUCTIONThe presence of soluble salts in porous materials is a problem frequently encountered by conservators treating archaeological objects. Because of their potential for damaging artifacts, the salts often have to be removed through desalination, whether by immersion, washing, or poulticing with water. This treatment can be problematic when the surface of the object is very fragile or when water-sensitive inks, pigments, or binders are present. In such instances, the surfaces of the artifacts have generally been consolidated, commonly with a resin, prior to desalination, to stabilize these areas (Strahan 1996; Koob 1991). Although consolidation may protect the object, it can sometimes alter the surface, making it dark or shiny, and can ultimately be difficult to completely remove after treatment. Cyclododecane (CDD), a volatile binder, has been primarily used in conservation as a temporary consolidant, barrier, or fixative for a variety of materials during treatment or transport. It is cyclododecane's potential application as a fixative or hydrophobic barrier over water-sensitive materials such as inks, dyes, pigments, and binding media (Hangleiter 1998; Bandow 1999; Bl�her et al. 1999; Br�ckle et al. 1999) that is the focus of this study. Research in the field of paper conservation in particular has shown the effectiveness of cyclododecane as a temporary fixative for ink on paper during washing. Not only have the hydrophobic properties of this material been useful in these aqueous treatments, but also the fact that it sublimes at room temperature ensures that the fixing is temporary. For this study, the previous use of cyclododecane as a temporary fixative was modified for a series of tests that examined the effectiveness of the barrier produced in protecting water-sensitive ink on archaeological ceramics during desalination. The aim of this research was to examine the working properties of CDD and determine which films would protect the ink on modern terracotta samples during a mock desalination. The results of these tests would then be applied to the desalination of an Egyptian ostracon, an inscribed terracotta sherd (fig. 1). It is hoped that the research conducted will help in determining
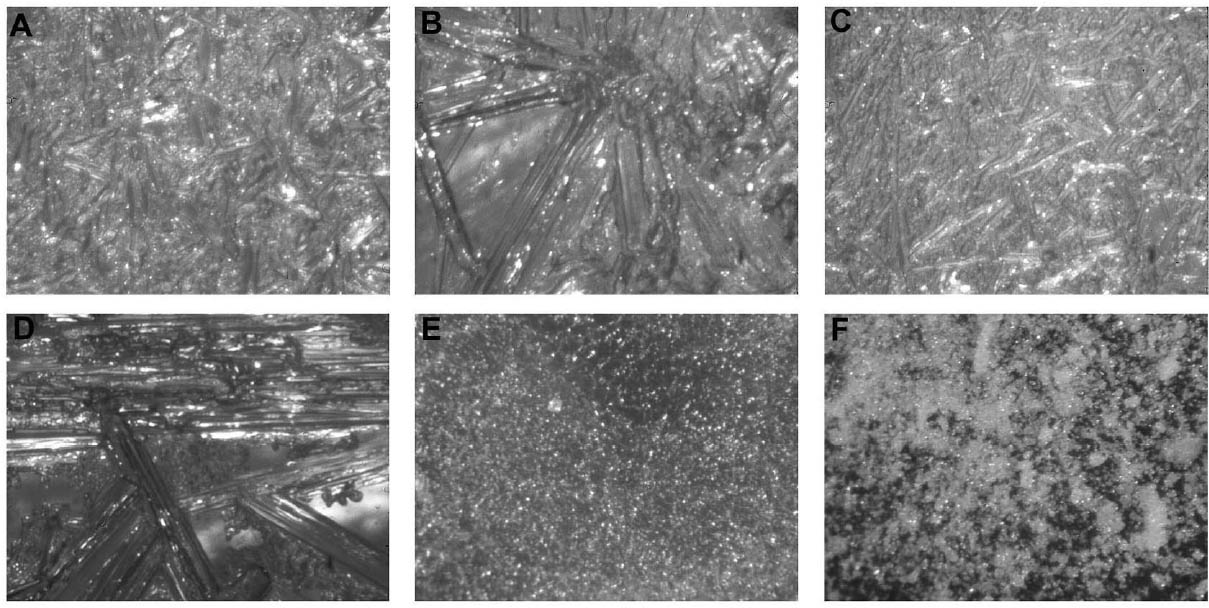
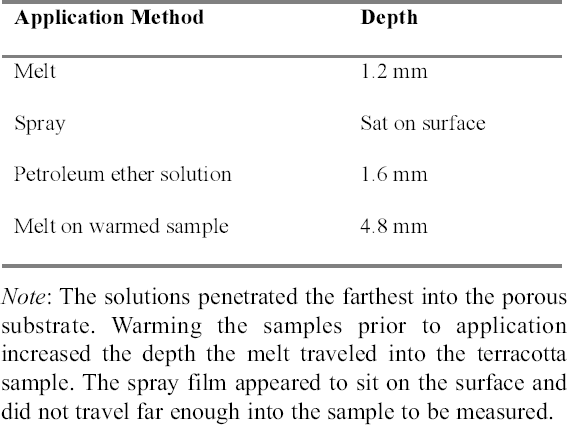
2 WORKING PROPERTIES OF CYCLODODECANEPrior to beginning any of the tests on modern samples, the working properties of CDD were examined. Several published studies (Br�ckle et al. 1999; Hangleiter 1999; Stein et al. 2000) discuss the structure of formed films of CDD using different application methods and how the application methods affect the material's properties and behavior. Many of the published examinations and tests that examined CDD's working properties were repeated for this study (appendix 1), some with slight modifications, to ensure that the same results would be obtained using a different substrate or additional application methods. The results of these studies were used to determine the protocol for the bench tests on modern terracotta samples and to focus the testing on those application methods expected to produce the best protective barriers for the water-sensitive ink. 2.1 APPLICATION METHODSCyclododecane (C12H24) is a cyclic hydrocarbon that appears as a waxy solid. It has a melting temperature of 58–61�C (J�egers and J�egers 1999), and with low heat can be applied to surfaces as a melt. It is soluble in a range of nonpolar solvents, allowing it to be applied in solution. It can also be purchased as an aerosol spray (Hangleiter 1999).1 The application method used can affect the structure of the CDD films produced, which in turn affects the behavior of the film. It is important for conservators to be aware of the characteristics of CDD films in order to determine which application method will produce the most suitable film for the desired treatment and end result. Previously published examinations (Br�ckle et al. 1999; Stein et al. 2000) have found that melting CDD on a glass slide produced a dense, compact layer of small acicular crystals. Solvent applications produced films with an open network of these crystals. The faster the solvent evaporated, the smaller and more tightly packed the crystalline structure formed. Added to the authors' examinations (fig. 2) was the aerosol spray film, which was made up of very small, slightly rounded crystals with spaces in between (appendix 1.1). From these evaluations it seems that the most effective, and least permeable, barrier would be made by those application methods that produce denser films with smaller crystals tightly packed together, such as the melt film or CDD in fast-evaporating solvent solutions. The spaces visible in some CDD solution-based films or the aerosol spray film may potentially allow water to permeate the CDD barrier and damage water-sensitive ink. 2.2 PENETRATION INTO POROUS SUBSTRATESTests have found that the application method can affect how deep CDD can penetrate into the substrate to which it is applied. Stein et al. (2000) learned that when comparing stone samples coated with cyclododecane as a melt and in solution, the solution tended to travel farther into the samples than the melt did. Warming samples prior to coating with molten CDD can help increase the depth of penetration
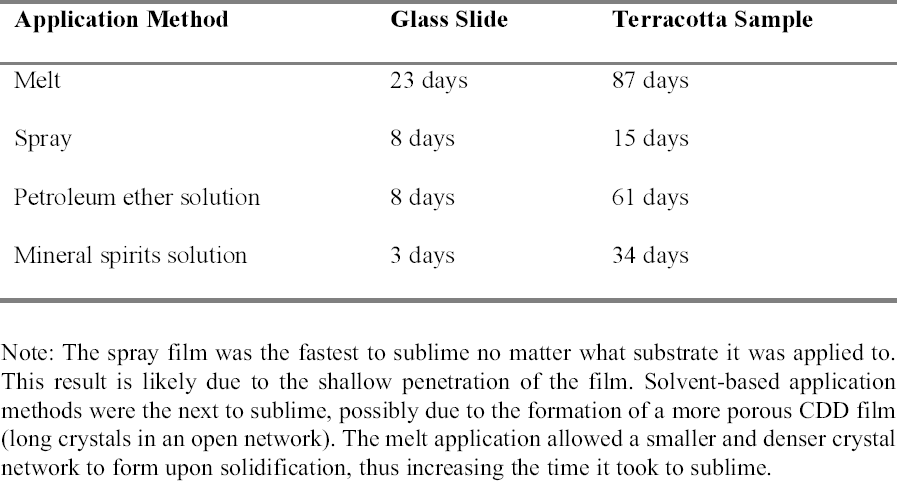
The results obtained were similar to those previously published (table 1). The aerosol spray, added to the authors' tests, appeared only to sit on the surface, and the depth of penetration could not be measured. For the purposes of this study, the CDD would need to travel far enough into the sample to completely coat or encapsulate the inscription so that no damage could occur even from the action of water underneath, or behind, the inscribed area. Since the aerosol spray film appeared to sit on the surface, it was thought that this application method would not produce an adequate barrier because it would not coat the inscription sufficiently. CDD as a melt or in a solvent solution could be used since both seemed to penetrate deep enough into the samples to completely coat any ink on the surface. 2.3 SUBLIMATION RATEThe sublimation rate of CDD has been found to be greatly affected by the method of application used, in addition to factors such as the surrounding air temperature, humidity, and ventilation (Hiby 1997). In tests where limestone and sandstone samples were coated with cyclododecane, the coating formed from the melt took two to three times longer to sublime than those applied in solvent form (Stein et al. 2000).
Examining the pore structure of plaster samples coated with CDD using a cryo-scanning electron microscope (SEM), Riedl and Hilbert (1998) found that a film of CDD from solution took five months to completely sublime, while the melt can take approximately nine months to completely sublime. Sublimation tests similar to those conducted by Stein et al. (2000) were repeated applying CDD on glass slides and terracotta samples (appendix 1.2). The sublimation rate of the aerosol spray film was included in these tests. The authors' results were similar to those of the investigations discussed above (table 2). The aerosol spray film was the first to sublime, possibly due to its superficial penetration exposing it more to the air, and to its less dense film formation. The solutions were the next to sublime, where CDD in petroleum ether took longer to sublime than the mineral spirits solution. This is perhaps due to the differences in the formation of the solid films. The film produced by the melt took the longest to sublime because it forms as a very dense film of small, tightly packed crystals. In treatments that may occur over long periods of time, it is important to understand the sublimation rate of the application method chosen to ensure that the CDD film is not compromised. 2.4 RESIDUESThough cyclododecane sublimes over time, recent analyses have found that some residues can remain within the coated material (Caspi and Kaplan 2001). Using various analytical techniques, residues of materials chemically related or similar to cyclododecane have been found within ceramics and on the surfaces of glass slides. The residues appear to come from impurities in CDD as a result of the manufacturing process. Caspi and Kaplan also found that the
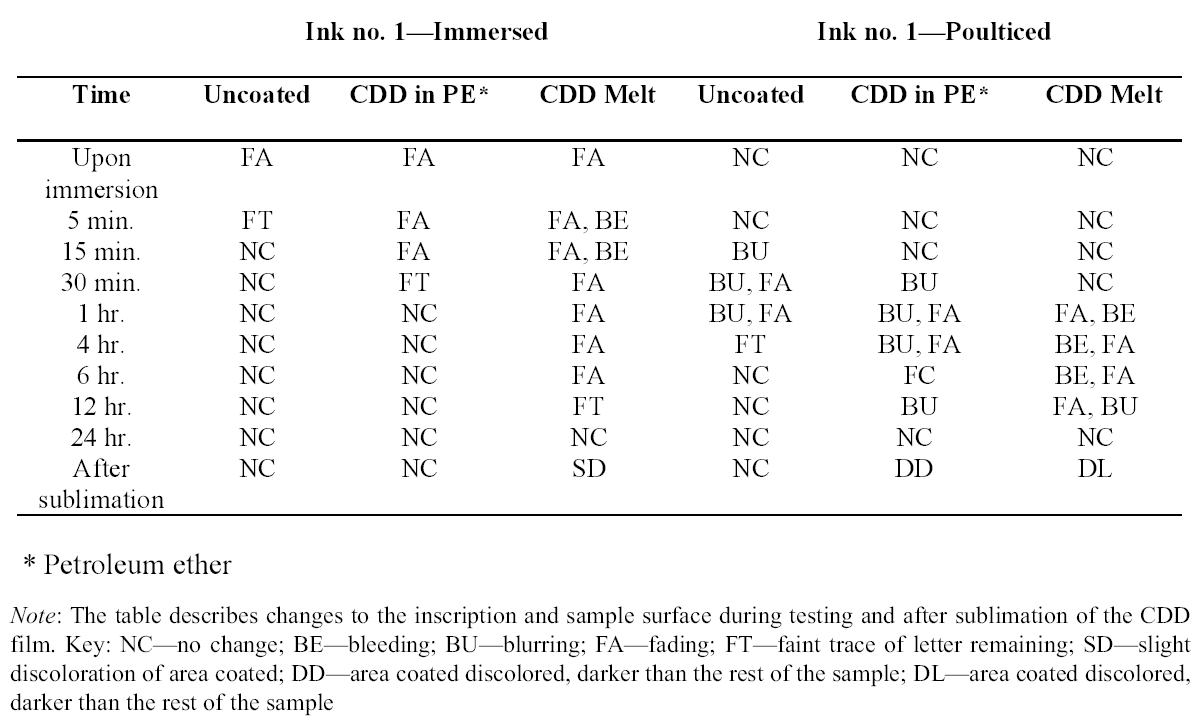
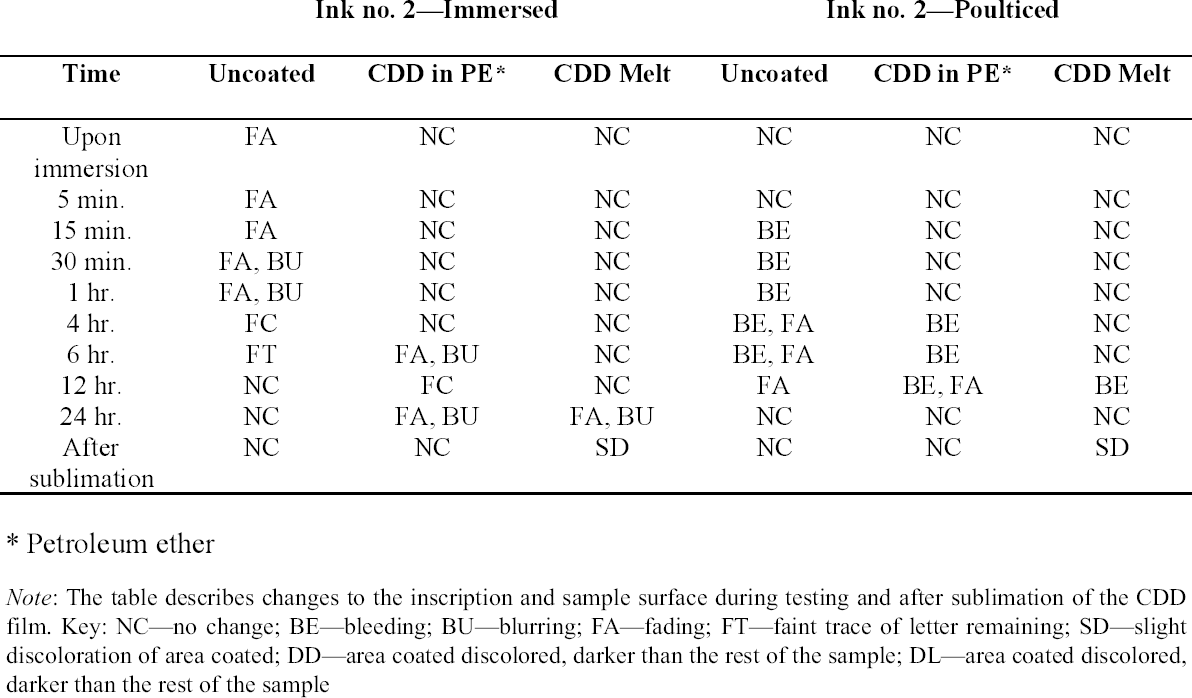
Tests were conducted by the authors to determine whether the batch of CDD used for these tests contained residues. Glass slides were coated with CDD as a melt, in various solutions and from the aerosol spray, based on the methodology of previous residue tests (Caspi and Kaplan 2001; Maish and Risser 2002). All slides exhibited white tidemarks after the sublimation of all the CDD films. Glass slides coated with only solvents also produced these tidemarks, likely due to impurities in the solvents. Therefore, the tidemarks seen on the slides are the result of impurities in the CDD and/or solvents, depending on the application method. Tidemarks like those seen on the slides were not visible on the surfaces of the terracotta samples coated for earlier examinations, but it is assumed residues are present within the samples. Testing and treatment using CDD will continue based on the conclusions of Caspi and Kaplan (2001). Future investigations and analysis, however, should be encouraged to further determine the effects of these residues, if any, on objects coated with cyclododecane. 3 TESTS USING MODERN TERRACOTTA SAMPLES3.1 SAMPLE PREPARATION AND TESTINGBefore CDD could be used for the desalination of the Egyptian osctracon, the material was tested on inscribed modern terracotta samples, in a variation of tests conducted by Br�ckle et al. (1999) of ink on paper. These tests would help to determine whether CDD would be effective at protecting water-sensitive ink during desalination and what application method would produce the best barrier. Based on the results of the examinations into CDD's working properties and published successful treatments, the melt and CDD in petroleum ether (PE) (90% w/v) would be the application methods used for testing. Prior to coating, the terracotta samples were marked with ink of two varying water sensitivities. One set was marked with a Paper Mate nylon fiber point pen (ink no. 1), which seemed to be very sensitive to water. The other set was marked with Higgins Fountain Pen India nonwaterproof black ink (ink no. 2), which was less affected by water. In addition to their water sensitivity, the inks were also tested to see if they were sensitive to petroleum ether. Neither was affected by the application of the solvent. Each of the samples, measuring about 7 mm thick, was coated with a 0.05–1 mm film of CDD. None of the samples were warmed during application, and they were kept at room temperature during testing. An uncoated sample was included for each ink type to see the effect of the desalination techniques on the unprotected ink. Two different desalination techniques were used for testing to determine if either had any effect on the behavior of the barrier films and inks. One set of samples from each ink type was immersed in deionized water, and another set was poulticed, using cotton wool and deionized water, both for 24 hours. The poultice material was applied to the back of the sample to easily observe any changes in the inscription. The inscriptions were examined at various intervals within a 24-hour period, after desalination and after sublimation of the CDD from the surface. 3.2 RESULTSThe CDD films were unable to completely protect the inscriptions marked with ink no. 1 (nylon fiber point pen) from damage (figs. 3, 4). The ink, whether coated by the melt or by petroleum ether solution, bled and faded. The extent of the damage observed was dependent more on the method of desalination used (table 3). The ink on the coated, immersed samples had a greater tendency to fade, while the ink on the poulticed samples was more likely to bleed. Cyclododecane proved to be ineffective at protecting this particular ink. Cyclododecane, however, was very effective at protecting ink no. 2 (nonwaterproof India ink) during both poulticing and immersion (figs. 5, 6). While the inscription on the uncoated samples bled and faded, the
The results of these tests concur with the results of treatments using CDD on works of art on paper, where the melt was found to be a more effective barrier than the solutions during aqueous treatments (Bandow 1999; Br�ckle et al. 1999). Br�ckle et al. (1999) also found, as was the case with the two inks tested in this study, that CDD films differ in their effectiveness as a barrier according to the ink's degree of sensitivity to water. The films did not protect ink no. 1, despite the application method, because the ink seemed to be extremely sensitive to water. The CDD barriers better protected ink no. 2, which was less sensitive to water. These differences in the behavior of cyclododecane CDD on different types of inks suggest that some idea of a particular ink's sensitivity to water should be known prior to using CDD during any aqueous treatments. The water sensitivity of the ink on the ostracon will have to be determined prior to treatment to help reduce the risk of any damage to the inscription. Surface examination of the samples, after sublimation of CDD from the surface, revealed a slight discoloration on areas that had been coated with cyclododecane. The surfaces coated with the melt appeared lighter than the rest of the sample. Under microscopic examination (10–40x magnification), these areas appeared rougher and more porous than the areas that were not coated with cyclododecane. Samples coated with the PE solution had darkened
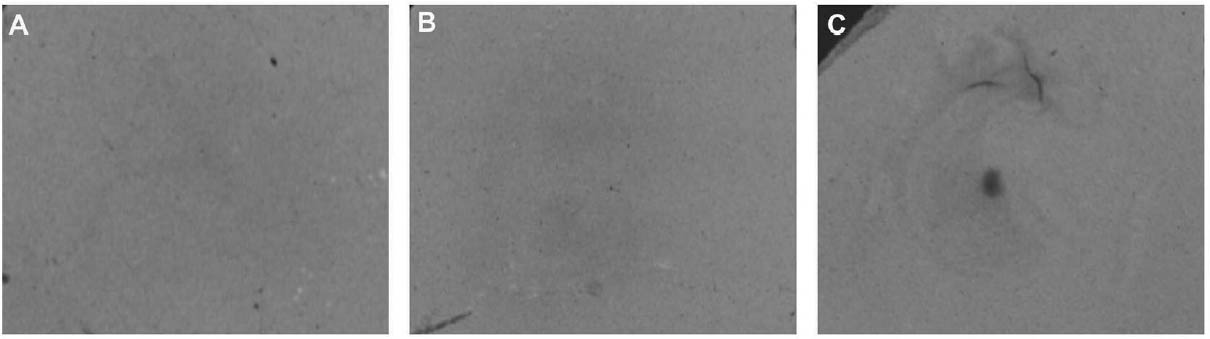
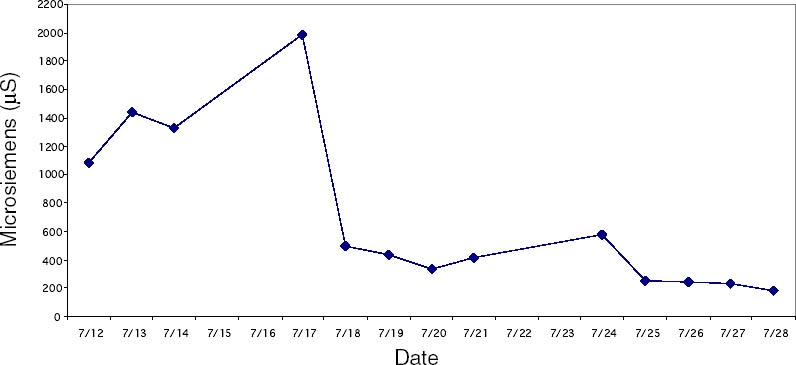
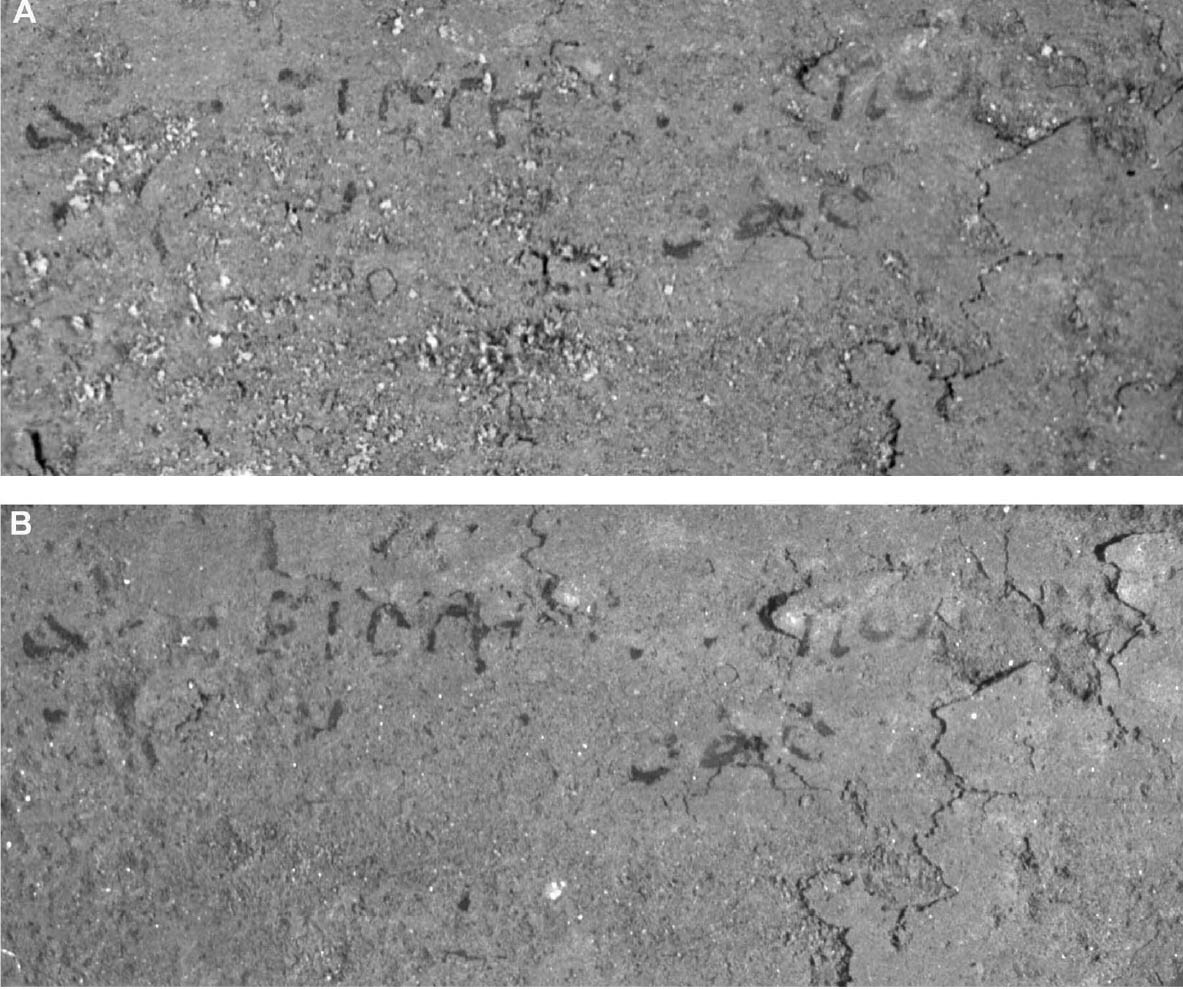
Since not all of the test samples exhibited these types of discoloration, other factors besides the application of CDD may be causing the surface changes. Because samples previously coated for earlier examinations, which were not soaked or poulticed, did not exhibit any surface changes, it is thought the discoloration or changes in surface texture may have been due to the dissolution of soluble material in the untreated areas of the terracotta samples during the mock desalination. Solvent residues or incomplete sublimation of CDD from the samples could also be factors in the discoloration. The cause and nature of the discoloration are areas that will need further investigation. 4 CASE STUDY: THE DESALINATION OF AN EGYPTIAN OSTRACONBased on the results of the tests on modern inscribed ceramic samples, CDD was used in the treatment of an ancient Egyptian ostracon that required desalination. The ostracon, in the collection of the Los Angeles County Museum of Art, suffered from a soluble salt problem. Having been previously stored in an environment with fluctuating temperature and humidity, the salts continued to crystallize and deliquesce within the pore structure, causing subsequent damage to the object. The surface of the ostracon was slightly flaky and powdery, with salt crystals visible. The salt damage was resulting in the loss of material, but, more important, in the loss of the inscription. A decision was made to desalinate the object, but because the ink or binder used was likely to be water sensitive, the inscription had to be protected first. CDD was chosen for the barrier because, in contrast Literature that discusses the writing of ancient Egypt (Forbes 1965; Singer et al. 1965) states that early inks were generally carbon black mixed with a binder, such as a gum or proteinaceous material, or in later periods an iron oxide–based material. In both cases, either the ink or the binder is considered water sensitive. A test was conducted to determine whether the ink, or binder, if present, was water sensitive. This test was also important because the age of the ink could cause it to behave differently than expected when in contact with water. To make these determinations, a small, disassociated flake with a small dot of ink was immersed in 30 ml of deionized water for 72 hours. The ink faded slightly upon immersion, but no other changes were observed. It was concluded that the ink was only slightly sensitive to water, and, therefore, based on earlier test results, the melt film should provide adequate protection for the inscription. A sample of the wash water used to observe the ink's sensitivity to water was used to determine what soluble salts were present. Microchemical tests were conducted to identify the presence of three common soluble salts in the wash water: silver nitrate to identify chlorides, ferrous sulfate for nitrates, and barium chloride for sulfates (Buys and Oakley 1993). The tests gave positive readings for the presence of nitrate and chloride salts. The CDD was melted on the surface of the ostracon using a Zephytronics Air Pencil ZT-2 (SMT Pin Point Soldering System), because it was felt the heated spatula, used for earlier tests, would apply too much pressure on the fragile surface. The air pencil was originally designed for soldering electronic and computer components, but it has been modified for use in conservation treatments, such as tape removal from paper (Stanley 1998). It melts or softens materials using warm air, where the air flow and temperature can be adjusted separately, as needed. The use of the air pencil would allow the cyclododecane to be melted using controllable warm air, without applying pressure to the object's surface. A low heat setting and the minimum airflow were used to melt the CDD on the ostracon. During application, pieces of solid cyclododecane were held just above the surface of the ostracon with tweezers. The air pencil was then held far enough away over the cyclododecane to allow the solid piece to melt, but not for the airflow to disturb any of the surface flakes. Only the area of the inscription was coated. The ostracon was desalinated by poulticing because immersion would result in the displacement of any flakes on the surface. Cotton wool wadding, dampened with deionized water, was placed behind the ostracon, where the surface was more cohesive. Poulticing from the back allowed the inscription to be visible during treatment so that any changes could be easily observed. The poultice material was changed every 24 hours, and the salt content monitored by soaking the poultice in 250 ml of deionized water and measuring the resulting conductivity. The ostracon was kept in a covered tray and sealed in a polyethylene bag during treatment to prevent the sublimation of the coating, something that occurred on some of the poulticed terracotta samples during testing. Desalination was completed after two months, when a series of similar conductivity readings were obtained within the acceptable level of 75–100 μS/cm (Buys and Oakley 1993) (fig. 7). The ostracon was left to dry out slowly in an open polyethylene bag, for about four to five weeks, until no cyclododecane remained on the surface and the inscription could be examined. Based on the sublimation rates from earlier tests, it is likely that some CDD remained within the ostracon and would require a few months to completely volatilize. Examination of the surface and inscription after treatment showed no bleeding or fading of the ink (fig. 8). The inscription appeared clearer after desalination. Comparison of the inscription before and after treatment showed no loss of the characters (fig. 9). No tidemarks were left, and no discoloration of
5 CONCLUSIONSThis study into the properties of cyclododecane has provided further insight into its hydrophobicity and a possible new use for this material in the treatment of archaeological objects. Adapting CDD's use as a temporary fixative for ink on art on paper during aqueous treatments, tests were developed and carried out to investigate the effectiveness of CDD as a temporary barrier for inscribed archaeological ceramics during desalination. Published examinations, and the authors' observations into the working properties of CDD, were used to define the parameters for a series of tests conducted on modern terracotta samples undergoing mock desalination. The samples tested, marked with two inks of different water sensitivities, showed that the application method used and the ink's sensitivity to water affected the behavior of the CDD film and how well the inscriptions were protected. The very water-sensitive ink was not protected by any of the CDD films. Both CDD in a petroleum ether solution and as a melt did create a better barrier for the less water-sensitive ink. The CDD melt film proved to be the most effective film in protecting inscriptions made with slightly water-sensitive ink from water damage during mock desalination. Based on these results, the desalination of an Egyptian ostracon was undertaken with successful results. This study is a preliminary investigation into this modified use of CDD and, it is hoped, will add to the ever-increasing body of knowledge concerning this material. These results may provide conservators with an alternative to the consolidation of pigments or inks on ceramics with acrylic resins or other materials that may be more permanent or difficult to completely remove, or which may alter the aesthetics of the object treated. This study has only begun to look into this particular use of cyclododecane, and, although further research is needed into the application of CDD to the desalination of inscribed archaeological objects, the results are promising.
ACKNOWLEDGEMENTSThe authors would like to thank Nancy Thomas, deputy director for curatorial affairs, and Victoria Blyth Hill, director of the Conservation Center at the Los Angeles County Museum of Art, for giving us the opportunity to conduct this research; Sara Caspi, conservator in private practice, for taking the time to discuss her CDD research; Brice Cantrell of the Department of Germanic Studies, University of Chicago, for his translation of the German articles; Alison Whyte, assistant conservator at the Oriental Institute Museum, for reading over and commenting on the early drafts; and Erik Risser, assistant conservator, Antiquities Conservation Department, J. Paul Getty Museum, for sharing his experiences with the use of CDD and for his editorial suggestions on the numerous versions of this article. NOTES1. No details are given of the exact nature of the propellant or solvent in the aerosol spray can since it is proprietary information. The information available on the website (www.hangleiter.com) states that in the spray can, cyclododecane is present in a solved form with the propellant being the only solvent. The solvent is described as being extremely volatile. APPENDIXAPPENDIX 1. METHODS USED FOR THE EXAMINATION OF CDD'S WORKING PROPERTIES1. Examination of Application Methods and Crystalline structure Cyclododecane was applied in various forms on glass slides to compare the crystalline structures produced by different application methods. The application methods used were CDD as a melt, in solutions, and in an aerosol spray. A total of nine solvents were used to make up the solutions, for testing: petroleum ether, naphtha, isooctane, mineral spirits, dichloromethane, toluene, xylene, 1-1-1, trichloroethane, and heptane. These solvents were chosen based on their uses during similar published examinations, as well as by simply choosing previously untested solvents in which CDD was soluble. The degree of solubility of cyclododecane in each solvent varied, and therefore all solutions were made to the same concentration, 85% w/v, for consistency during testing. For the melt film, a heated spatula was used. A spraying distance of at least 3–4 cm was used for the aerosol spray, as recommended by Hangleiter (1999). The slides were examined using a binocular microscope, 10–40x magnification. Some of the films formed from the various application methods examined are shown in figure 2. 2. Determination of the Sublimation of CDD from Glass Slides and Terracotta Samples Sublimation rate tests were conducted in a method similar to that used by Maish and Risser (2002) and Stein et al. (2000). Glass slides and several terracotta samples, measuring 3.5 x 4 cm and 7 mm thick, were coated for these tests. The surfaces of the samples were treated with cyclododecane as a melt, a spray, in petroleum ether, and in mineral spirits. Cyclododecane was applied until a 2 mm film was formed. The samples were kept uncovered and at room temperature (23�C, 40% RH) during testing. The sublimation process was monitored by weighing the samples before coating and then reweighing them every 24 hours until they reached their pretreatment weight. It was assumed that at this point, the cyclododecane would have sublimed completely from the sample. REFERENCESBandow, C.1999. Cyclododecan in der Papierrestaurierung. Restauro5:314–19. Bl�her, A., A.Haverditzel, and T.Wimmer. 1999. Aqueous conservation treatment of 20th century papers containing water-sensitive inks and dyes. Restaurator20:181–97. Br�ckle, I., J.Thornton, K.Nichols, and G.Strickler. 1999. Cylcododecane: Technical note on some uses in paper and objects conservation. Journal of the American Institute for Conservation38:162–75. Buys, S., and V.Oakley. 1993. The conservation and restoration of ceramics.Oxford: Butterworth-Heinemann. Caspi, S., and E.Kaplan. 2001. Dilemmas in transporting unstable ceramics: A look at cyclododecane. In Objects Specialty Group Postprints, vol. 8, 2001, ed. V.Greene and L.Bruno. Washington D. C.: American Institute for Conservation of Historic and Artistic Works. 116–35. Forbes, R. J.1965. Studies in ancient technology, vol. 3. Leiden: E. J. Brill. Hangleiter, H. M.1998. Erfahrungen mit fl�chtigen Bindemitteln. Restauro5:314–19. Hangleiter, H. M.1999. Volatile binding media. www.hangleiter.com/Bindemittel/e_bindeframeset.htm (accessed October 2002).
Hiby, G.1997. Das fl�chtige Bindemittel Cyclododecan. Restauro2:96–103. J�egers, E., and E.J�egers. 1999. Volatile binding media: Useful tools for conservation. In Reversibility: Does it exist?British Museum occasional paper no. 135, ed. A.Oddy and S.Carroll. London: British Museum Press. 37–42. Koob, S. P.1991. The use of Acryloid B-72 in the treatment of archaeological ceramics: Minimal intervention. In Material issues in art and archaeology, II. Materials Research Society Symposium Proceedings vol. 185, ed. P. B.Vandiver et al. Pittsburgh: Materials Research Society. 591–96. Kumar, R., and W. S.Ginell. 1997. A new technique for determining the depth of penetration of consolidants into limestone using iodine vapor. Journal of the American Institute for Conservation36:143–50. Maish, J. P., and E.Risser. 2002. A case study in the use of cyclododecane and latex rubber in the molding of marble. Journal of the American Institute for Conservation41:127–38. Riedl, N., and G.Hilbert. 1998. Cyclododecan in Putzgef�ge. Restauro7:494–99. Singer, C., E. J.Holmyard, and A. R.Hall. 1965. A history of technology. Vol. 1: From early times to the fall of ancient empires. Oxford: Clarendon Press. Stanley, T.1998. A tool for pressure sensitive tape removal: The AirPencil. The Book and Paper Group Annual, vol. 17. http://aic.stanford.edu/conspec/bpg/annual/v17/bp17-16.html (accessed October 2002). Stein, R., J.Kimmel, M.Marincola, and F.Klemm. 2000. Observations on cyclododecane as a temporary consolidant for stone. Journal of the American Institute for Conservation39:355–69. Strahan, D. K.1996. Preserving unstable painted surfaces on freshly excavated terracotta: Dilemmas and decisions. In Archaeological conservation and its consequences, ed. A.Roy and P.Smith. London: International Institute for Conservation of Historic and Artistic Works. 172–76. SOURCES OF MATERIALSColorhue Instant Set silk dyesThings Japanese 9805 NE 116th St. Kirkland, Wash. 98034 CyclododecaneKremer Pigments Inc. 228 Elizabeth St. New York, N.Y. 10012 Cyclododecane—spray canHans-Michael Hangleiter GmbH Bismarckstrasse 13 D64853 Otzberg Germany Higgins Fountain Pen India nonwaterproof black ink, item 46030 (723)Sanford Bellwood, Ill. 60104 Paper Mate nylon fiber point pen, Series 860, black ink, no. 863-11The Gillette Co. Paper Mate Division Box 61 Boston, Mass. 02199 Zephytronics AirPencil ZT-2SMT Pin Point Soldering System 225 N. Palomares Pomona, Calif. 91767 AUTHOR INFORMATIONVANESSA MUROS is an assistant conservator at the Oriental Institute Museum, University of Chicago. After receiving her B.A. in archaeology from Boston University, she completed the master's program in conservation at the Institute of Archaeology, University College, London. She has been an intern in the Metals Conservation Section at the British Museum and has worked as a conservator on various excavations. The research conducted for this article was completed during her Mellon Fellowship in Objects Conservation at the Los Angeles County Museum of Art in 2000. Address: Oriental Institute Museum, University of Chicago, 1155 E. 58th St., Chicago, Ill. 60637 JOHN HIRX is the head objects conservator at the Los Angeles County Museum of Art. For almost 10 years, he has worked in both conservation research and objects conservation, examining, analyzing, and treating works of art. In particular, he has focused on conservation and analytical studies related to ancient silver, Islamic ceramics, and seismic mitigation of LACMA's monumental sculptures. He has served as vice chair and chair for the Research and Technical Studies (RATS) specialty group of AIC. Prior to working at LACMA, Hirx worked for the Brooklyn Museum of Art, where he treated objects in the Egyptian collection prior to reinstallation, and for the Metropolitan Museum of Art. Address: Los Angeles County Museum of Art, 5905 Wilshire Blvd., Los Angeles, Calif. 90036
 Section Index Section Index |