HUNTING FREE AND BOUND CHLORIDE IN THE WROUGHT IRON RIVETS FROM THE AMERICAN CIVIL WAR SUBMARINE H. L. HUNLEY
N�STOR G. GONZ�LEZ, PHILIPPE DE VIVI�S, MICHAEL J. DREWS, & PAUL MARDIKIAN
4 SUMMARY AND CONCLUSIONS
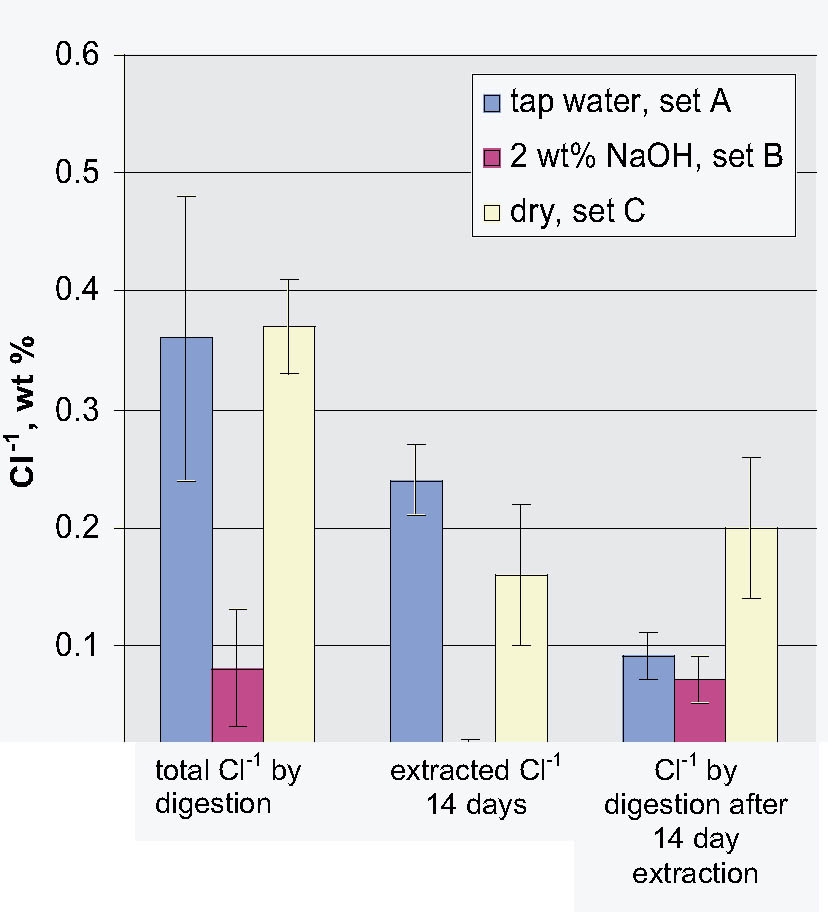
This article addresses the issue of the presence of free and bound chloride in the metal shavings retained when the hull plate rivets were drilled as part of their removal process, prior to the excavation of the Hunley's interior. Before the extraction and digestion studies on the shavings were begun, one of the rivets that had been stored in tap water since its removal was sectioned. The sectioned surface was characterized using scanning electron microscopy and energy dispersive x-ray analysis. This analysis showed the general nature of the corrosion processes that had occurred in the rivet over nearly 140 years. Of particular interest was the presence of inclusions containing high levels of Cl-1 (> 50,000 ppm). Rivet shavings that had been stored dry, in tap water, and in 2 wt% NaOH immediately after removal were extracted with deionized water, 2.5 wt% Na2CO3, and 1 and 2 wt% NaOH solutions for up to two weeks. The total amount of chloride present in each sample was determined using nitric acid digestion. It was found that the shavings stored dry and in tap water contained very similar quantities of total chloride, ~ 0.4 wt%. It was also found that the shavings stored in caustic solution contained significantly less chloride than the other two sets of samples, less than 0.1 wt%. For all of the shaving samples, it was found that a significant quantity of chloride (> 500 ppm) remained in the corrosion products after extraction.
Some of the more significant conclusions that may be drawn from these results are summarized below:
- Based on the results of the digestion experiments, it was clearly shown that the storage solution had a significant effect on the total Cl-1 content of the shavings. It was also found that the shavings stored in caustic solution contained significantly less chloride than the other two sets of samples. However, since the shavings stored in tap water and dry had similar Cl-1 contents, this finding could not be due solely to a difference in dry or solution storage.
- It would appear that for these shaving samples, the extraction efficiency was the same regardless of whether the samples were extracted with DI water, Na2CO3, or NaOH solutions. Consequently, these results would appear to suggest that counterion replacement was playing little or no role in the extraction under these experimental conditions.
- No statistically significant difference was observed between the 72-hour and 14-day extractions. This result suggests that there are clearly at least two different classes of Cl-1 present in these samples, one class that is very labile and easy to extract and another that is very difficult to remove. Whether this second class can ever be completely removed by extraction alone is a question that needs to be addressed.
- As is clearly shown once again by this combined extraction and digestion study, simply relying on measurements of the amount of Cl-1 removed from an artifact does not reflect in any manner on the Cl-1 remaining in it. For example, in this study, extraction of the shavings stored in tap water removed 0.24 wt% and those stored in NaOH 0.01 wt%, yet digestion of the remaining solids for both contained ~ 0.08 wt% or ~ 800 ppm.
Work is in progress to address some of the questions
Fig. 7.
Comparison of the total wt% Cl-1 to the extractable and residual weight % Cl-1 data from the 14-day extraction and digestion experiments
 |
posed by the results of this investigation. Mossbauer and x-ray diffraction studies have been initiated in an attempt to more fully characterize the nature of the corrosion products present in these shaving samples. A similar set of extraction and digestion studies has been initiated using the rivets themselves. These experiments will include electrolysis or plasma reduction as part of the extraction protocol. Finally, alternative approaches to determining the total Cl-1 content of these samples without complete digestion are being investigated.
ACKNOWLEDGEMENTS
The authors would like to thank the Department of Defense (through the DOD Legacy funds), the state of South Carolina (Hunley Commission), and the Friends of the Hunley for supporting this research. We are especially indebted to Bob Neyland, Hunley Project director, Warren Lasch, chairman of the Friends of the Hunley, and Maria Jacobsen, senior archaeologist, for their support and to all the personnel of the Warren Lasch Conservation Center for their assistance. One of the authors would also like to especially thank Clemson's School of Materials Science and Engineering and the College of Engineering and Science for allowing his participation in this research as well as the partial financial support for this work received from Clemson University. We would like to thank Linda Jenkins of Clemson's Department of Bioengineering for her assistance in sectioning the rivet sample, Bill Kay of Clemson's Electron Microscope facility for the electron micrographs of the rivet sections, and Kim Ivey of the School of Materials Science and Engineering for her assistance in sample preparation.
|