TECHNICAL EXAMINATION OF RENAISSANCE MEDALS THE USE OF LAUE BACK REFLECTION X-RAY DIFFRACTION TO IDENTIFY ELECTROFORMED REPRODUCTIONSGlenn Wharton
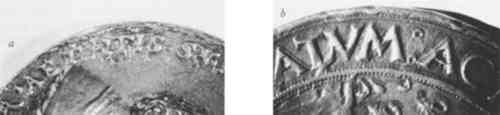
2 DISTINGUISHING METHODS OF FABRICATION BY VISUAL EXAMINATIONTHE SURFACE OF a cast structure will frequently have small pits from air bubbles which were trapped as the metal solidified (Fig. 2a). Filing and chasing marks may appear where gates and vents were formerly attached, or where imperfections in the cast were reworked. Close examination may also reveal cast dendritic crystals on the surface which have preferentially corroded. Struck medals, on the other hand, can often be identified by sharp detail with no undercutting around the relief. At times they exhibit chatter marks from multiple strikes, and edge cracking from stress (Fig. 2b). Although a cast copy of a struck medal may reproduce these features, they are not usually as sharp as those on an original struck piece.
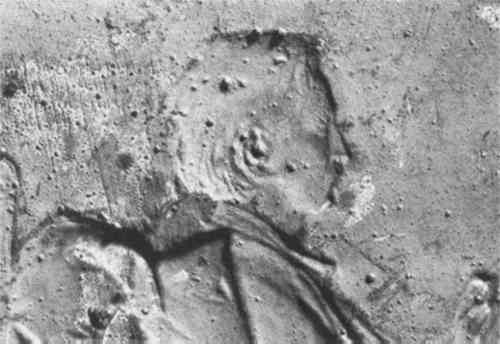
In composition, Renaissance medals are usually bronze or brass.5 After the fifteenth century, gold and silver came into use as well. Lead medals were also produced, often as trial proofs for final casts.6 Early electrotypes were always made in pure metal: most commonly copper, but also silver, nickel and even gold. Alloys were not used in the beginning because of the difficulty in preventing one metal from plating out preferentially to the other. All of the variables in the process, including bath composition, temperature, polarity around the electrodes, voltage, and current density had to be carefully monitored to obtain an alloy deposit. Yet by 1876 several patents had been taken out for the deposition of alloys, and it was becoming increasingly popular in industry.7 However, most art reproductions continued to be made in pure metal. The medals may have been patinated either with pigmented varnishes or by chemical treatment. The varnishes, which were most popular during the Renaissance, range from transparent to opaque. They consist of pigments suspended in binders such as linseed oil, sandarac gum, pitch, alum or wax.8 Chemical patination, popular If an electrotype was to remain one-sided, it was commonly filled with lead or lead/tin solder on the reverse to give it substantial weight. One-sided electrotypes which were not filled with lead can be identified on the reverse either by the exact negative of relief from the obverse or by their characteristic nodular texture (Fig. 3). These nodules grow as a result of uneven depositing on preferred sites called “active areas,” or “growth areas.” Once initiated, these protuberances attract more current than surrounding areas and grow at a faster rate.10
If two electrotypes were to be joined to create a two-sided medal, they were soldered together and the join was filed, chased, and patinated. Usually this join line is visible on close examination. If these characteristic features cannot be detected, other techniques of distinguishing medals may be utilized. |